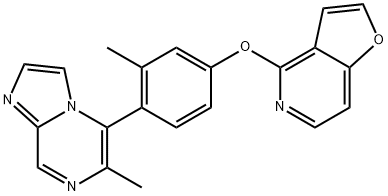
Imidazo[1,2-a]pyrazine, 5-[4-(furo[3,2-c]pyridin-4-yloxy)-2-methylphenyl]-6-methyl-, (5R)- synthesis
- Product Name:Imidazo[1,2-a]pyrazine, 5-[4-(furo[3,2-c]pyridin-4-yloxy)-2-methylphenyl]-6-methyl-, (5R)-
- CAS Number:1609583-15-4
- Molecular formula:C21H16N4O2
- Molecular Weight:356.39
Yield:1609583-15-4 91%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium carbonate in 1,4-dioxane;water; for 10 h;Reflux;Inert atmosphere;
Steps:
25.1; 26.1 Step 1. Synthesis of 5-[4-(furo[3,2-c]pyridin-4-yloxy)-2-methylphenyl]-6-methylimidazo[1,2-a]pyrazine (C46).
To a solution of 4-[3-methyl-4-(4,4,5,5-tetramethyl-1 ,3,2-d ioxaborolan-2- yl)phenoxy]furo[3,2-c]pyridine (C2) (13.5 g, 38.4 mmol) in 1,4-dioxane (200 mL) and water (10 mL) were added 5-bromo-6-methylimidazo[1,2-a]pyrazine (C45, see A. R. Harris et al.,Tetrahedron 2011, 67, 9063-9066) (8.15 g, 38.4 mmcl), potassium carbonate (15.9 g, 115 mmol) and [1 ,1’-bis(diphenylphosphino)ferrocene]dichloropalladium(ll) (2.8 g, 3.8 mmol) at room temperature. The reaction mixture was degassed with nitrogen for 5 minutes, then stirred for 10 hours at reflux. The mixture was cooled to room temperature and filtered; the filtrate wasconcentrated in vacuo and purified via chromatography on silica gel (Gradient: 0% to 50% ethyl acetate in petroleum ether) to afford the product as a yellow solid. Yield: 12.4 g, 34.8 mmol, 91%. LCMS m/z 357.0 (M+H). 1H NMR (400 MHz, CD3OD) ? 9.02 (s, 1H), 8.00 (d, J6.0 Hz, 1H), 7.93 (d, J=2.0 Hz, 1H), 7.79-7.80 (m, 1H), 7.48-7.51 (m, 1H), 7.44 (d, J=8.5 Hz, 1H), 7.41 (dd, J=6.0, 1.0 Hz, 1H), 7.36 (br d, J=2.0 Hz, 1H), 7.28 (br dd, J=8, 2 Hz, 1H), 7.02-7.05 (m,1H), 2.38 (s, 3H), 2.07 (s, 3H).
References:
WO2014/72881,2014,A1 Location in patent:Page/Page column 102; 103
![4-CHLOROFURO[3,2-C]PYRIDINE](/CAS/GIF/31270-80-1.gif)
31270-80-1
115 suppliers
$10.00/100mg
![Imidazo[1,2-a]pyrazine, 5-[4-(furo[3,2-c]pyridin-4-yloxy)-2-methylphenyl]-6-methyl-, (5R)-](/CAS/20211123/GIF/1609583-15-4.gif)
1609583-15-4
2 suppliers
inquiry
14472-14-1
266 suppliers
$10.00/5g
![Imidazo[1,2-a]pyrazine, 5-[4-(furo[3,2-c]pyridin-4-yloxy)-2-methylphenyl]-6-methyl-, (5R)-](/CAS/20211123/GIF/1609583-15-4.gif)
1609583-15-4
2 suppliers
inquiry
![Furo[3,2-c]pyridine, 4-(4-bromo-3-methylphenoxy)-](/CAS/20210305/GIF/1609581-00-1.gif)
1609581-00-1
0 suppliers
inquiry
![Imidazo[1,2-a]pyrazine, 5-[4-(furo[3,2-c]pyridin-4-yloxy)-2-methylphenyl]-6-methyl-, (5R)-](/CAS/20211123/GIF/1609583-15-4.gif)
1609583-15-4
2 suppliers
inquiry
![5-bromo-6-methylimidazo[1,2-a]pyrazine](/CAS/GIF/1346157-10-5.gif)
![Furo[3,2-c]pyridine, 4-[3-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenoxy]-](/CAS/20200611/GIF/1609581-01-2.gif)