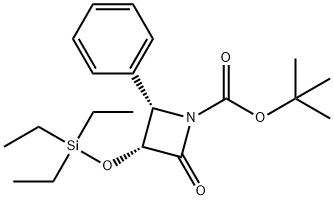
(3R,4S)-tert-Butyl 2-oxo-4-phenyl-3-(triethylsilyloxy)azetidine-1-carboxylate synthesis
- Product Name:(3R,4S)-tert-Butyl 2-oxo-4-phenyl-3-(triethylsilyloxy)azetidine-1-carboxylate
- CAS Number:149198-47-0
- Molecular formula:C20H31NO4Si
- Molecular Weight:377.55
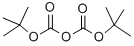
24424-99-5
833 suppliers
$13.50/25G
![(3R-cis)-4-Phenyl-3-[(triethylsilyl)oxy]-2-azetidinone](/CAS/GIF/149140-54-5.gif)
149140-54-5
33 suppliers
inquiry

149198-47-0
160 suppliers
$140.00/25mg
Yield:149198-47-0 2.4 g (Y: 83%)
Reaction Conditions:
with dmap;N-ethyl-N,N-diisopropylamine in tetrahydrofuran;ethyl acetate;
Steps:
12 (3R,4S) -1-t-Butoxycarbonyl-4-phenyl-3-triethylsilyloxy-2-azetidinone (IVa) STR12
EXAMPLE 12 (3R,4S) -1-t-Butoxycarbonyl-4-phenyl-3-triethylsilyloxy-2-azetidinone (IVa) STR12 To a stirred solution of (3R,4S)-4-phenyl-3-triethylsilyloxy-2-azetidinone (XXII) (2.200 g, 7.92 mmol) in dry THF (25 mL) was added N,N-diisopropylethylamine (1.65 mL. 9.510 mmol, 1.2 equiv) at 0° C. under an argon atmosphere. The solution was stirred for 5 min followed by the addition of di-t-butyldicarbonate (2.080 g, 9.510 mmol, 1.2 equiv) and 4-dimethylaminopyridine (193.6 mg, 1.581 mmol, 0.20 equiv). The reaction mixture was stirred at 0° C. for 60 min. The solution was diluted by adding ethyl acetate (25 mL). The resulting solution was washed with brine, 10% NaHC3, 10% HCl solution, dried (MgSO4), and concentrated to give a crude compound (oil). The compound was further purified by silica gel flash chromatography (eluted with 15% ethyl acetate in hexanes) to afford 2.4 g (Y: 83%) of the title β-lactam as a white solid; 1 H-NMR (CDCl3) δ7.28 (m, 5H) 5.03 (m, 2H) 1.39 (s, 9H) 0.76 (t, J=7.6 Hz, 9H) 0.43 (m, 6H).
References:
US5478854,1995,A
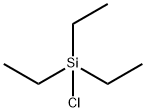
994-30-9
422 suppliers
$12.00/5g

149198-47-0
160 suppliers
$140.00/25mg
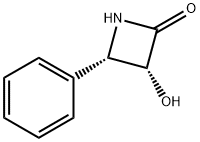
132127-34-5
174 suppliers
$17.00/100mg

149198-47-0
160 suppliers
$140.00/25mg
![2-Azetidinone, 4-phenyl-3-[[tris(1-methylethyl)silyl]oxy]-, (3R,4S)-](/CAS/20210305/GIF/132127-31-2.gif)
132127-31-2
0 suppliers
inquiry

149198-47-0
160 suppliers
$140.00/25mg