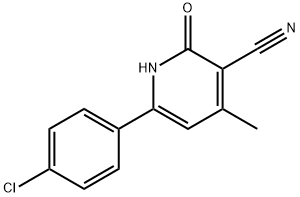
6-(4-CHLOROPHENYL)-1,2-DIHYDRO-4-METHYL-2-OXOPYRIDINE-3-CARBONITRILE synthesis
- Product Name:6-(4-CHLOROPHENYL)-1,2-DIHYDRO-4-METHYL-2-OXOPYRIDINE-3-CARBONITRILE
- CAS Number:134600-02-5
- Molecular formula:C13H9ClN2O
- Molecular Weight:244.68
Yield:134600-02-5 56%
Reaction Conditions:
Stage #1: 1-(4'-chlorophenyl)-1,3-butanedione;malononitrilewith diethylamine at 20 - 25; for 19 - 51 h;Heating / reflux;
Stage #2: with hydrogenchloride;water at 20; for 0.5 h;
Steps:
I.a.1.b; A.22.1
Step 1 protocol b (R1 is alkyl): To a mixture of a 1,3-diketo-compound of formula III (wherein R1 is alkyl; prepared from the corresponding acetophenone of general formula I and the R1-carboxylic acid derivative under conditions as e.g. described in general procedure I step 1, Synthesis 1991, (3), 195; Monatsh. Chem. 1996, 127-(8-9), 895-907; Angew. Chem. Int. Ed. Engl. 1993, 32(8), 1151-1152; J. Org. Chem. 1993, 58(11), 3185-3187; J. Fluorine Chem. 1986, 32(2), 229-231; Org. Synth. Coll. Vol. III, 387; J. Am. Chem. Soc. 1953, 75, 626 and 4109; J. Am. Chem. Soc. 1941, 63, 2785; Chem. Ber. 1970, 103, 1088) and malononitrile (1.33 eq.) in a protic solvent (e.g. ethanol) at ambient temperature is added a catalytic amount of diethylamine (0.2 eq.) and the mixture was stirred at 20 to 25° C. for around 3 h. Then the mixture was heated under reflux conditions for around 16 to 48 h. After cooling to room temperature, the mixture was diluted with 1M aqueous HCl, stirred for 30 min, the precipitate was filtered off, washed with ethanol and was dried in air at 60° C. overnight to give the crude product, which was purified by trituration with ethanol/diethyl ether/acidic acid to give the pure product (according to J. Indian Chem. Soc. 1930, 7, 815).; EXAMPLE A.222-Bromo-6-(4-chloro-phenyl)-4-methyl-pyridine1) 6-(4-Chloro-phenyl)-4-methyl-2-oxo-1,2-dihydro-pyridine-3-carbonitrile: The compound was prepared from commercially available ethyl acetate, commercially available 4-chloro-acetophenone and commercially available malononitrile according to the general procedure Ia step 1 protocol b. Obtained as a yellow solid (56%). MS (ISP) 245.5 [(M+H)+] and 247 [(M+2+H)+].
References:
US2007/232583,2007,A1 Location in patent:Page/Page column 24; 25; 33
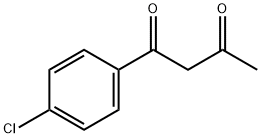
6302-55-2
43 suppliers
$45.00/50mg
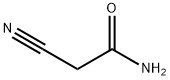
107-91-5
547 suppliers
$5.00/10g

134600-02-5
6 suppliers
inquiry
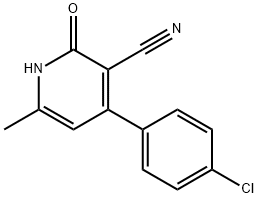
134600-03-6
0 suppliers
inquiry
