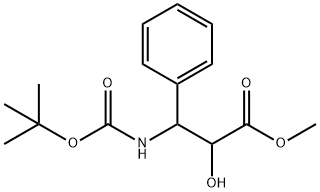
Methyl (2R,3S)-3-(tert-butoxycarbonylamino)-2-hydroxy-3-phenylpropionate synthesis
- Product Name:Methyl (2R,3S)-3-(tert-butoxycarbonylamino)-2-hydroxy-3-phenylpropionate
- CAS Number:124605-42-1
- Molecular formula:C15H21NO5
- Molecular Weight:295.33
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

124605-42-1
176 suppliers
$8.00/250mg
Yield: 99%
Reaction Conditions:
with hydrogen;1% Pd/C in methanol under 4654.46 Torr;
Steps:
24
Example 24. Methyl (2/?,3S)-2-hydroxy-3-fert-butoxy-carbonylamino-3- phenylpropanoate (33).; To a solution of methyl (2R,3S)-2-benzyloxy-3-te/t- butoxy-carbonylamino-3-phenylpropanoate (32; 55 mg, 0.14 mmol) in MeOH (4 ml_), palladium on carbon (5 mg) was added. The reaction mixture was stirred under H2 (90 psi) atmosphere. When the starting material could no longer be detected by TLC, the solution was filtered through celite and concentrated to give the title compound as white crystals in 99% yield (42 mg). 1H NMR (400 MHz, CDCI3): δ = 7.38-7.18 (m, 5H), 5.44 (d, J=9.6Hz, 1 H), 5.22 (d, J=8.8Hz, 1 H), 4.46 (bs, 1 H), 3.83 (s, 3H), 1.40 (s, 9H); 13C NMR (100 MHz, CDCI3): 173.6, 155.4, 139.3, 128.8, 128.0, 126.9, 80.2, 73.8, 56.3, 53.3, 28.5; HRMS (ESI): calcd for [M+Na] (Ci5H2iNO5) requires m/z 318.1312, found 318.1313; [α]D = + 13.2 (c=1.0, CHCI3).
References:
ORGANOCLICK AKTIEBOLAG;CORDOVA, Armando;DZIEDZIC, Pawel;VESELY, Jan WO2010/24762, 2010, A1 Location in patent:Page/Page column 19-20
24424-99-5
839 suppliers
$13.50/25G
157240-36-3
16 suppliers
inquiry

124605-42-1
176 suppliers
$8.00/250mg

24424-99-5
839 suppliers
$13.50/25G
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

124605-42-1
176 suppliers
$8.00/250mg

67-56-1
756 suppliers
$7.29/5ml-f

24424-99-5
839 suppliers
$13.50/25G
132201-32-2
213 suppliers
$6.00/250mg

124605-42-1
176 suppliers
$8.00/250mg

24424-99-5
839 suppliers
$13.50/25G

124605-42-1
176 suppliers
$8.00/250mg