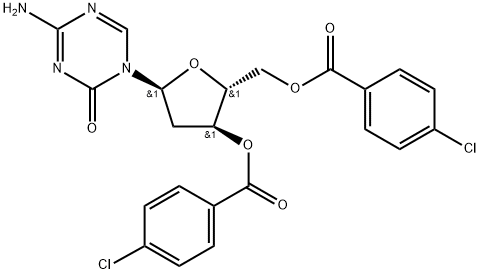
4-Amino-1-[3,5-bis-O-(4-chlorobenzoyl)-2-deoxy-α-D-erythro-pentofuranosyl]-1,3,5-triazin-2(1H)-one synthesis
- Product Name:4-Amino-1-[3,5-bis-O-(4-chlorobenzoyl)-2-deoxy-α-D-erythro-pentofuranosyl]-1,3,5-triazin-2(1H)-one
- CAS Number:1140891-02-6
- Molecular formula:C22H18Cl2N4O6
- Molecular Weight:505.31
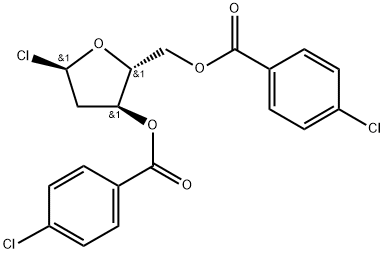
21740-23-8
228 suppliers
$21.00/1g
![N-(Trimethylsilyl)-4-[(trimethylsilyl)oxy]-2-amine-1,3,5-triazin](/CAS/GIF/52523-35-0.gif)
52523-35-0
31 suppliers
inquiry
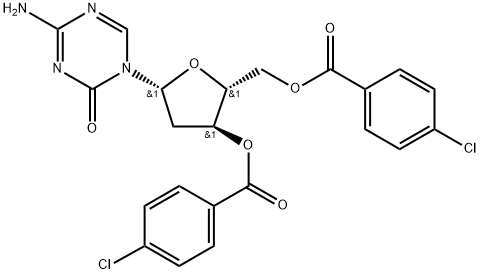
1034301-08-0
130 suppliers
$190.00/25mg
![4-Amino-1-[3,5-bis-O-(4-chlorobenzoyl)-2-deoxy-α-D-erythro-pentofuranosyl]-1,3,5-triazin-2(1H)-one](/CAS/20211123/GIF/1140891-02-6.gif)
1140891-02-6
34 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 3,5-O-bis(4-chlorobenzoyl)-2-deoxy-α-D-ribofuranosyl chloride;2-(trimethylsilylamino)-4-(trimethylsilyloxy)-s-triazinewith trimethylsilyl trifluoromethanesulfonate in chloroform at 1; for 21 h;Inert atmosphere;
Stage #2: with sodium hydrogencarbonate in chloroform;water; pH=6;
Steps:
6
Example 6 Preparation of 4-amino-1-[3,5-di-O-(p-chlorobenzoyl)]-2-deoxy-alpha-D-ribofuranosyl-1,3,5-triazin-2(1H)-one and 4-amino-1-[3,5-di-O-(p-chlorobenzoyl)]-2-deoxy-beta-D-ribofuranosyl-1,3,5-triazin-2(1H)-one.Purified 5-azacytosine (1.62 g), ammonium sulfate (0.162 g) and hexamethyldisilazane (40 mL) were charged into a 4-neck, 100 mL round-bottom flask under nitrogen. The mixture was heated to 117° C. under stirring and kept at this temperature for 2 hours to give a clear solution. Unreacted hexamethyldisilazane was removed under vacuum to give 2-[(trimethylsilyl)amino]-4-[(trimethylsilyl)oxy]-s-triazine as a white solid. The solid was dissolved in anhydrous chloroform (25 mL) and was added into a solution of 1-[3,5-di-O-(p-chlorobenzoyl)]-2-deoxy-alpha-D-ribofuranosyl-chloride (5.0 g, from Example 2) in anhydrous chloroform and stirred at 1° C. under nitrogen. Trimethylsilyl trifluoromethanesulfonate (1.0 g) in anhydrous chloroform (10 mL) was added and the resulting mixture was stirred at this temperature for 21 hours. Saturated aqueous sodium bicarbonate was added to neutralize to pH 6. The mixture was diluted with additional chloroform (100 mL). After phase cut, the organic layer was washed with brine (30 mL) and dried over magnesium sulfate. The mixture was filtered through a bed of filter aid. Methanol was added and the solution was concentrated to 20 mL followed by the addition of anhydrous hexanes (40 mL) under stirring to form slurry. The solid was collected and dried under vacuum to give the product (5.06 g) that contains a mixture of 2.5:1 beta:alpha anomers. It is ready to be used in a process according to this invention for preparing Decitabine, without further purification.
References:
US2010/249394,2010,A1 Location in patent:Page/Page column 5