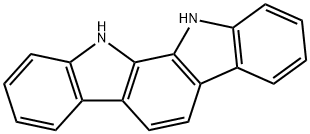
11,12-Dihydroindolo[2,3-a]carbazole synthesis
- Product Name:11,12-Dihydroindolo[2,3-a]carbazole
- CAS Number:60511-85-5
- Molecular formula:C18H12N2
- Molecular Weight:256.3
![11,12-DIHYRDOINDOLO[2,3-A]CARBAZOLE 11,12-DIHYRDOINDOLO[2,3-A]CARBAZOLE](/NewsImg/2023-09-11/6383003895736949039297168.jpg)
Yield:60511-85-5 92.1%
Reaction Conditions:
in decalin; for 0.5 h;Reflux;
Steps:
1 Embodiment 1
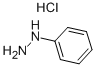
To 1L three-mouth flask add decahydronaphthalene in 500 ml, 1, 2 - Cyclohexanedione 22.4g, phenyl hydrazine hydrochloride 57.6g, heating control temperature (190 °C ± 2), 30 minutes later, stopping the reaction, solvent recovery is used the reaction process. Cooling to room temperature, and a large amount of precipitation, filtering, to obtain the powder (1) 11, 12 - indoline and [2,3 - the a] carbazole 47.2g, (yield of 92.1%, HPLC=99.7%).
References:
CN106478635,2017,A Location in patent:Paragraph 0021-0023
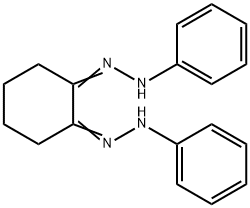
6782-77-0
0 suppliers
inquiry
![11,12-Dihydroindolo[2,3-a]carbazole](/CAS/GIF/60511-85-5.gif)
60511-85-5
219 suppliers
$30.00/1g
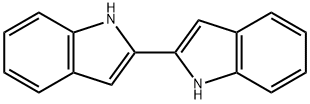
40899-99-8
20 suppliers
$205.00/100mg
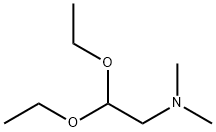
3616-56-6
208 suppliers
$5.00/5g
![11,12-Dihydroindolo[2,3-a]carbazole](/CAS/GIF/60511-85-5.gif)
60511-85-5
219 suppliers
$30.00/1g

![Indolo[3,2-b]carbazole](/CAS/GIF/6336-32-9.gif)