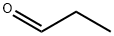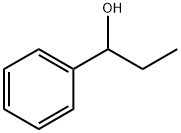
1-Phenyl-1-propanol synthesis
- Product Name:1-Phenyl-1-propanol
- CAS Number:93-54-9
- Molecular formula:C9H12O
- Molecular Weight:136.19
Yield:93-54-9 89%
Reaction Conditions:
Stage #1:bromobenzene with n-butyllithium in tetrahydrofuran;hexane at -78; for 0.5 h;
Stage #2:propionaldehyde in tetrahydrofuran;hexane at -78; for 1 h;
Steps:
Synthesis of 1-phenyl-1-propanol
To a flame dried 50 mL round bottomed flask equipped with a magnetic stir bar, 0.32 mL (3 mmol) of bromobenzene in 15 mL dry THF was added at -78 oC. Then, n-butyllithium (1.32 mL, 3.3 mmol, 2.5 M in hexane or PAO432) was added dropwise and allowed to react for 30 minutes, at which time propionaldehyde (0.26 mL, 3.6 mmol) in 15 mL dry THF was added dropwise. After 1 h, the reaction was quenched with 20 mL saturated ammonium chloride. The phases were separated, and the aqueous phase was washed once with 15 mL hexane. The combined organic phases were washed once with 15 mL 2 M HCl. The organic phase was then dried over sodium sulfate, filtered, and the solvent was removed under reduced pressure. The residue was then taken up in 10 mL hexane and extracted once with 10 mL MeCN. Solvent from the MeCN phase was then removed via reduced pressure to give the product. (89 % yield hexanes, 85 % yield PAO) 1H NMR (500 MHz, CDCl3) δ=7.25-7.38 (m, 5H), 4.60 (t, J= 6.5 Hz, 1H), 1.90 (bs, 1H), 1.79 (m, 2H), 0.92 (t, J=7.5 Hz, 3H) 13C NMR (125 MHz, CDCl3) δ=144.6, 128.4, 127.5, 125.97, 76.0, 31.9, 10.1
References:
Malinski, Thomas J.;Bergbreiter, David E. [Tetrahedron Letters,2018,vol. 59,# 44,p. 3926 - 3929] Location in patent:supporting information
4393-06-0
32 suppliers
inquiry

93-54-9
244 suppliers
$6.00/5g

93-55-0
7 suppliers
$15.00/25g

93-54-9
244 suppliers
$6.00/5g
4187-87-5
171 suppliers
$9.00/1g

93-54-9
244 suppliers
$6.00/5g