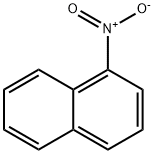
1-Nitronaphthalene synthesis
- Product Name:1-Nitronaphthalene
- CAS Number:86-57-7
- Molecular formula:C10H7NO2
- Molecular Weight:173.17
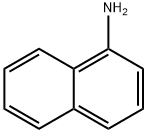
134-32-7
373 suppliers
$9.00/1g

86-57-7
7 suppliers
$14.00/5g
Yield:86-57-7 90%
Reaction Conditions:
with trifluoroacetyl peroxide in dichloromethane at -5 - 5; for 4 h;
Steps:
6
Add a peroxytrifluoroacetic acid solution to the reaction vessel, lower the temperature to -5 to 5 ° C, add 1-aminonaphthalene 859.14g (6mol), keep the temperature at -5 to 5 ° C, and stir the reaction for 4 hours. After the reaction, add 2L of water and stir , And allowed to stand and separate. The organic layer was washed with 2L saturated sodium bisulfite solution, 2L saturated sodium bicarbonate solution, and water to pH 7-8, and the solvent was distilled off under reduced pressure.With dichloromethane and methanol (dichloromethane and methanol volume ratio of 1: 2)The solution was recrystallized to obtain 935.1 g (yield 90%) of as a yellow solid.
References:
Chongqing Pharmaceutical College;Liu Dianqing;He Dongxian;Wang Kun;Guo Shengchao;Wang Guangming CN110330432, 2019, A Location in patent:Paragraph 0044; 0048

90-14-2
290 suppliers
$19.00/5g

86-57-7
7 suppliers
$14.00/5g
91-20-3
511 suppliers
$13.19/250mg

86-57-7
7 suppliers
$14.00/5g
86-55-5
375 suppliers
$5.00/5g

86-57-7
7 suppliers
$14.00/5g
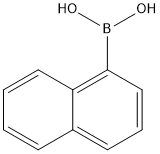
13922-41-3
497 suppliers
$5.00/1g

86-57-7
7 suppliers
$14.00/5g