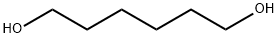
1,6-Hexanediol synthesis
- Product Name:1,6-Hexanediol
- CAS Number:629-11-8
- Molecular formula:C6H14O2
- Molecular Weight:118.17
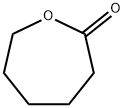
502-44-3
344 suppliers
$5.00/25g
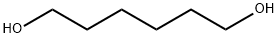
629-11-8
448 suppliers
$6.00/25g
Yield:629-11-8 98%
Reaction Conditions:
with C21H35BrMnN2O2P;hydrogen;potassium hydride in toluene at 100; under 15001.5 Torr; for 36 h;
Steps:
16
Hydrogenation of 1 mmol of hexyl hexanoate under 20 bar at 100°C in toluene, resulted in 99% yield of hexanol (Entry 1). Under the same conditions ethyl butyrate was hydrogenated to give 98% yield of butanol and 91 % yield of ethanol after 50 hours (Entry 2). When the reaction was performed at shorter reaction time (22 hours, Entry 2bis), small amounts of ethyl acetate and butyl butanoate were also formed, attributed to a transesterification reaction with the formed ethanol and butanol. Cyclohexylmethyl acetate gave 99% yield of cyclohexylmethanol and 60% yield of ethanol (Entry 3), and no transesterification products were observed. Hydrogenation of the secondary aliphatic ester heptan-2-yl acetate resulted in 98% yield of heptane-2-ol and 57% yield of ethanol (Entry 4). Ethyl 3-phenylpropanoate was smoothly hydrogenated, rendering 99% yield of 3-phenylpropan-l-ol and 70% yield of ethanol after 21 hours (Entry 5). Similarly, ethyl 3- phenylpropanoate gave 99% yield of phenyknethanol and 74% yield of butanol after 22 hours (Entry 6). In order to get full hydrogenation of benzyl benzoate longer reaction time was needed (43 hours, 99% yield benzyl alcohol, Entry 7). Similarly, methyl benzoate gave 96% yield of benzyl alcohol and 63 % of methanol after 50 hours (Entry 8). ε-Caprolactone was smoothly and quantitatively hydrogenated to 1 ,6-hexanediol (99% yield, Entry 9). The activated benzyl trifluoroacetate gave 99% yield of benzyl alcohol and 78% of 2,2,2- trifluoroethanol (Entry 10), and no secondary products where observed. Gratifyingly, allyl trifluoroacetate gave 97% yield of 2,2,2-trifluoroethanol and 96% of allyl alcohol (Entry 1 1), showing high chemoselectivity to ester hydrogenation over C=C hydrogenation. Hydrogenation of ethyl 4-isocyano-benzoate required an increase of precatalyst loading to 3%, probably due to competing nitrile coordination, and resulted in 61 % yield of (4-isocyanophenyl)methanol and 66% yield of ethanol, with no hydrogenation of the nitrile group detected (Entry 12).
References:
WO2017/137984,2017,A1 Location in patent:Paragraph 00352-00353
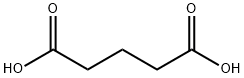
110-94-1
505 suppliers
$5.00/25g

124-04-9
1005 suppliers
$6.00/25g
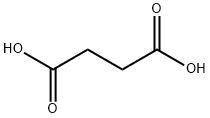
110-15-6
948 suppliers
$5.00/10g

111-29-5
364 suppliers
$5.00/25g
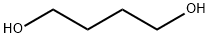
110-63-4
698 suppliers
$24.80/250g
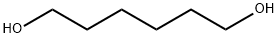
629-11-8
448 suppliers
$6.00/25g

124-04-9
1005 suppliers
$6.00/25g
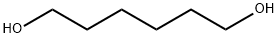
629-11-8
448 suppliers
$6.00/25g
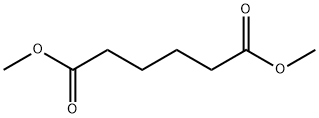
627-93-0
474 suppliers
$5.00/25g
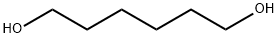
629-11-8
448 suppliers
$6.00/25g
