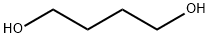
1,4-Butanediol synthesis
- Product Name:1,4-Butanediol
- CAS Number:110-63-4
- Molecular formula:C4H10O2
- Molecular Weight:90.12
The most prevalent 1,4-BD production route worldwide is BASF's Reppe process, which reacts acetylene and formaldehyde. Acetylene reacts with two equivalents of formaldehyde to form 1,4-butynediol, also known as but-2-yne- 1,4-diol. Hydrogenation of 1,4-butynediol gives 1,4-butanediol. 1,4-BD is also made on a large industrial scale by continuous hydrogenation of the 2-butyne- 1,4-diol over modified nickel catalysts. The one-stage flow process is carried out at 80 - 160 deg C and 300 bar.
Mitsubishi uses a three-step process:
(1) the catalytic reaction of butadiene and acetic acid yields 1,4-diacetoxy-2-butene;
(2) subsequent hydrogenation gives 1,4-diacetoxybutane; and
(3) hydrolysis leads to 1,4-butanediol.

106-65-0
475 suppliers
$6.00/25g
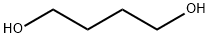
110-63-4
703 suppliers
$24.80/250g
Yield:110-63-4 99.4%
Reaction Conditions:
with hydrogen at 230; under 135014 Torr;Temperature;Reagent/catalyst;Pressure;
Steps:
1.1.2.b; 2.2.2.b; 3.3.2.b; 4.4.2.b b. Hydrogenation reaction
The separated dimethyl succinate was preheated at 80°C and passed into a fixed bed reactor, the reactor was equipped with an activated hydrogenation catalyst 40%Cu-15%Ni-5%Cr-10%Zn/SiO2.The reaction conditions are: hydrogen pressure 18MPa, temperature 230°C, dimethyl succinate liquid hourly space velocity 0.4h-1, and the molar ratio of hydrogen and dimethyl succinate is 300:1.The product stream consisted primarily of 1,4-butanediol, methanol, tetrahydrofuran, methyl 4-hydroxybutyrate, n-butanol, water and unreacted dimethyl succinate and hydrogen.The reaction product was analyzed by gas chromatography, and the conversion rate of dimethyl succinate was 99.2%, and the mass selectivity of 1,4-butanediol was 99.4%.After the product stream is separated by distillation, the intermediate product 1,4-butanediol is obtained with a purity of 99.0%.
References:
CN114539070,2022,A Location in patent:Paragraph 0093; 0102; 0105-0106; 0109; 0119; 0122-0123; ...

123-25-1
456 suppliers
$5.00/25g
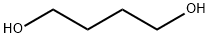
110-63-4
703 suppliers
$24.80/250g
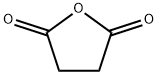
108-30-5
638 suppliers
$5.00/25g
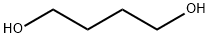
110-63-4
703 suppliers
$24.80/250g

98-01-1
484 suppliers
$22.00/25g
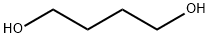
110-63-4
703 suppliers
$24.80/250g

110-65-6
323 suppliers
$13.00/50g
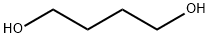
110-63-4
703 suppliers
$24.80/250g