
1,4-Dichlorobutane synthesis
- Product Name:1,4-Dichlorobutane
- CAS Number:110-56-5
- Molecular formula:C4H8Cl2
- Molecular Weight:127.01
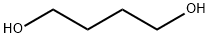
110-63-4
703 suppliers
$24.80/250g

110-56-5
311 suppliers
$15.00/25mL
Yield:110-56-5 97%
Reaction Conditions:
with hydrogenchloride;ammonium chloride in water at 56 - 105; for 3 h;
Steps:
3 Example 3 The preparation method of 1,4-dichlorobutane, the steps as follows:
Into a 1000ml reaction flask with oil-water separator and condenser,Put in 400 grams of water, 5 grams of ammonium chloride, and 322 grams of 1,4-butanediol in sequence, and stir and raise the temperature to 56°C;Start to pass HCL gas. When the clear and transparent reaction liquid in the reaction flask becomes milky white, continue to pass HCL gas, increase the temperature of the reaction liquid to 105°C, and start reflux and liquid separation;As the reflux reaction progresses, there is stratification in the oil-water separator. The reflux reaction is 3 hours, and the molar ratio of 1,4-butanediol to HCL gas is 1:2.6;The lower water phase is refluxed into the reaction flask, and the upper oil layer is 1,4-dichlorobutane separated and collected.The purity determined by HPLC was 99.5%, and the yield based on 1,4-dichlorobutane was 97%.
References:
CN112341309,2021,A Location in patent:Paragraph 0020
109-99-9
1055 suppliers
$9.00/5ml

110-56-5
311 suppliers
$15.00/25mL

109-99-9
1055 suppliers
$9.00/5ml

6334-96-9
105 suppliers
$24.69/5g

110-56-5
311 suppliers
$15.00/25mL

6334-96-9
105 suppliers
$24.69/5g

98-88-4
580 suppliers
$10.00/5g
946-02-1
64 suppliers
$28.60/250mg

110-56-5
311 suppliers
$15.00/25mL
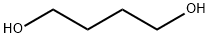
110-63-4
703 suppliers
$24.80/250g
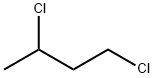
1190-22-3
76 suppliers
$39.00/1g

928-51-8
334 suppliers
$6.00/5g

6334-96-9
105 suppliers
$24.69/5g

110-56-5
311 suppliers
$15.00/25mL