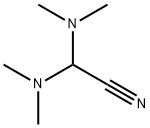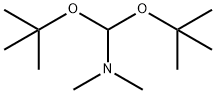
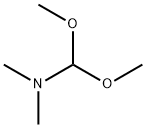
N,N-Dimethylformamide di-tert-butyl acetal synthesis
- Product Name:N,N-Dimethylformamide di-tert-butyl acetal
- CAS Number:36805-97-7
- Molecular formula:C11H25NO2
- Molecular Weight:203.32
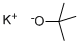
865-47-4
677 suppliers
$14.00/25g
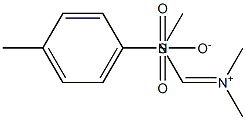
![[(dimethylamino)methylene]dimethylammonium methyl sulphate](/CAS/GIF/2013-91-4.gif)
2013-91-4
3 suppliers
inquiry
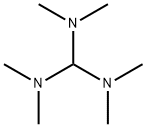
5762-56-1
70 suppliers
$102.00/1g
5815-08-7
168 suppliers
$6.00/1g

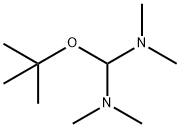
36805-97-7
154 suppliers
$29.00/1g
Yield:-
Reaction Conditions:
in tetrahydrofuran at 60; for 1 h;
Steps:
14.1
Example 14: Tris(dimethylamino)methane (13, R6 = Me, R7 = Me), Tert-Butoxy- bis(dimethylamino)methane (14, R6 = Me, R7 = Me, R8 = tBu) and N,N- Dimethylformamide di-tert-butyl acetal (15, R6 = Me, R7 = Me, R8 = tBu); Method 1; N,N,N',N'-tetramethylformadinium methyl sufate (5 g) (prepared according to Example 1, X = MeSO4) is added to a solution of potassium tert-butoxide in THF (23.6 ml, 1 M). The mixture is then stirred for 1 h at 60 °C. The reaction mixture is then filtered under argon. The mother liquor is then concentrated in vacuo to afford a residue (1.60 g) containing 13, 14 and 15 (R6 = Me, R7 = Me, R8 = tBu). 1H NMR (C6D6): 1.08, 1.16, 1.24, 2.29, 2.33, 3.02, 4.06, 5.00. Relative amounts of 13 (R6 = Me; R7 = Me), 14 (R6 = Me, R7 = Me, R8 =
References:
NOVARTIS AG WO2009/90251, 2009, A2 Location in patent:Page/Page column 143-144
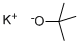
865-47-4
677 suppliers
$14.00/25g
3218-35-7
1 suppliers
inquiry

5762-56-1
70 suppliers
$102.00/1g

5815-08-7
168 suppliers
$6.00/1g

36805-97-7
154 suppliers
$29.00/1g
4637-24-5
607 suppliers
$5.00/25g
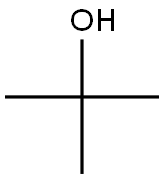
75-65-0
753 suppliers
$10.00/10ml

36805-97-7
154 suppliers
$29.00/1g

5815-08-7
168 suppliers
$6.00/1g

36805-97-7
154 suppliers
$29.00/1g