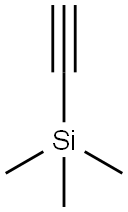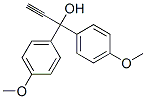
1,1-Bis(4-methoxyphenyl)-2-propyn-1-ol synthesis
- Product Name:1,1-Bis(4-methoxyphenyl)-2-propyn-1-ol
- CAS Number:101597-25-5
- Molecular formula:C17H16O3
- Molecular Weight:268.31
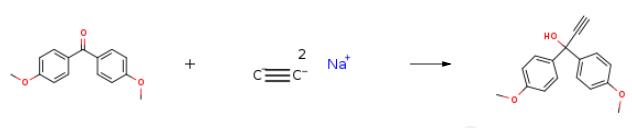
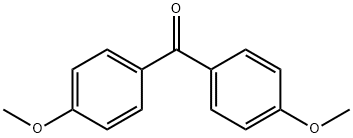
90-96-0
360 suppliers
$22.00/25g

4301-14-8
122 suppliers
$68.50/100ml

92298-55-0
3 suppliers
inquiry
Yield:92298-55-0 91%
Reaction Conditions:
in tetrahydrofuran at 25 - 60; for 2 h;Inert atmosphere;
Steps:
23 4.3.23. 1,1-Bis(4-methoxyphenyl)prop-2-yn-1-ol, (18g)
Ethynylmagnesium bromide (4 mmol, 0.5?M in THF) was added to the suspension of ketone 15 (0.5?g, 2.0?mmol) in THF (2?mL) at 25?°C.
The mixture was then stirred at 60?°C for 2?h and then quenched with saturated aqueous NH4Cl solution.
The aqueous layer was separated and extracted three times with Et2O.
The combined organic fractions were washed with brine, dried over Na2SO4 and concentrated under reduced pressure.
The crude product was recrystallized from Et2O/PE. White solid (490?mg, 91%).
Mp: 90-91?°C. IR (KBr): ?=?3472, 3245, 3004, 2959, 2932, 2836, 2545, 2102, 2039, 1933, 1902, 1886, 1870, 1683, 1635, 1607, 1584, 1506, 1464, 1455, 1440, 1415, 1350, 1305, 1295, 1251, 1241, 1199, 1180, 1164, 1199, 1180, 1164, 1119, 1062, 1025, 991, 967, 930, 897, 842, 882, 815, 782, 764, 732, 715, 685, 633, 627, 587, 526, 510, 488?cm-1. 1H NMR (500?MHz, CDCl3) δ 2.79 (s, 1H), 2.86 (s, 1H), 3.79 (s, 6H), 6.83-6.89 (m, 4H), 7.47-7.53 (m, 4H) ppm. 13C NMR (126?MHz, CDCl3) δ 55.42, 73.76, 75.22, 86.92, 113.68, 127.46, 137.06, 159.26?ppm. HRMS (EI): [M]+ calcd. for C17H16O3, 268.1099; found: 268.1096.
References:
Tóth, Krisztián;H?fner, Georg;Wanner, Klaus T. [Bioorganic and Medicinal Chemistry,2018,vol. 26,# 12,p. 3668 - 3687]

90-96-0
360 suppliers
$22.00/25g
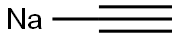
1066-26-8
90 suppliers
$48.00/50g

74-86-2
72 suppliers
inquiry

92298-55-0
3 suppliers
inquiry