Cobalt sulfate
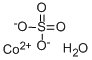
|
- $1334.03
- Product name: Cobalt sulfate
- CAS: 10124-43-3
- MF: CoO4S
- MW: 155
- EINECS:233-334-2
- MDL Number:MFCD00010943
- Synonyms:Cobalt (2+) sulfate;Cobalt sulfate (1:1);cobaltsulfate(1:1) ;cobaltsulfate(coso4) ;Sulfuric acid, cobalt(2+) salt (1:1);Sulfuricacid,cobalt(2+)salt(1:1) ;sulfuricacid,cobalt(2++)salt(1:1) ;COBALT ATOMIC SPECTROSCOPY STD. CONC.10. 00 G CO, AMPOULE
1 prices
Selected condition:
Brand
- American Custom Chemicals Corporation
Package
- 10G
- ManufacturerAmerican Custom Chemicals Corporation
- Product numberING0003977
- Product descriptionCOBALT SULPHATE 95.00%
- Packaging10G
- Price$1334.03
- Updated2021-12-16
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| American Custom Chemicals Corporation | ING0003977 | COBALT SULPHATE 95.00% | 10G | $1334.03 | 2021-12-16 | Buy |
Properties
Melting point :decomposes at 1140℃ [JAN85]
Density :d425 3.71
vapor pressure :0Pa at 20℃
refractive index :1.639
solubility :H2O: soluble
form :red orthorhombic crystals
color :red orthorhombic crystals, crystalline
Odor :Odorless
Water Solubility :dissolves slowly in boiling H2O [MER06]; g/100g solution H2O: 19.7 ± 0.1 (0°C), 27.2 ± 0.1 (25°C), 27.8 (100°C); solid phase, CoSO4 · 7H2O (0°C, 25°C), CoSO4 ·H2O (100°C) [KRU93]
Merck :13,2473
InChI :InChI=1S/Co.H2O4S.H2O/c;1-5(2,3)4;/h;(H2,1,2,3,4);1H2/q+2;;/p-2
InChIKey :BGORGFZEVHFAQU-UHFFFAOYSA-L
SMILES :S([O-])([O-])(=O)=O.O.[Co+2]
LogP :-1.031 (est)
CAS DataBase Reference :10124-43-3(CAS DataBase Reference)
NIST Chemistry Reference :Cobalt sulfate(10124-43-3)
EPA Substance Registry System :Cobalt(II) sulfate (10124-43-3)
Density :d425 3.71
vapor pressure :0Pa at 20℃
refractive index :1.639
solubility :H2O: soluble
form :red orthorhombic crystals
color :red orthorhombic crystals, crystalline
Odor :Odorless
Water Solubility :dissolves slowly in boiling H2O [MER06]; g/100g solution H2O: 19.7 ± 0.1 (0°C), 27.2 ± 0.1 (25°C), 27.8 (100°C); solid phase, CoSO4 · 7H2O (0°C, 25°C), CoSO4 ·H2O (100°C) [KRU93]
Merck :13,2473
InChI :InChI=1S/Co.H2O4S.H2O/c;1-5(2,3)4;/h;(H2,1,2,3,4);1H2/q+2;;/p-2
InChIKey :BGORGFZEVHFAQU-UHFFFAOYSA-L
SMILES :S([O-])([O-])(=O)=O.O.[Co+2]
LogP :-1.031 (est)
CAS DataBase Reference :10124-43-3(CAS DataBase Reference)
NIST Chemistry Reference :Cobalt sulfate(10124-43-3)
EPA Substance Registry System :Cobalt(II) sulfate (10124-43-3)
Safety Information
| Symbol(GHS): |
  
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Danger | |||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
The blue, crystalline hydrate Co2(SO4)3.18H2O is prepared by the oxidation of cobalt(II) sulphate in 8N sulphuric acid either electrolytically or chemically with ozone or fluorine. It is stable in the dry state, but is decomposed by water with evolution of oxygen; it is fairly stable in solution in dilute sulphuric acid. Cobalt(III) alums MCo(SO4)2.12H2O (M = K, Rb, Cs or NH4) can be isolated as blue crystals from the mixed cooler solutions of the two sulphates in dilute sulphuric acid. The potassium alum is diamagnetic, the rubidium salt has a magnetic moment less than 1 B.M. and the ammonium alum has a moment of 2.1 B.M. at 304°K. The hydrated sulphate also has a small positive magnetic susceptibility. The sulphate is believed like the alums to contain the [Co(H2O)6]3+ ion.Related product price
- Ammonium sulfate
$9-5423 - Aluminium sulfate
$16.8-1482.65 - Cobalt
$34.2-7990
Suppliers and manufacturers
Henan Tianfu Chemical Co.,Ltd.
Hefei TNJ Chemical Industry Co.,Ltd.
Hubei Jusheng Technology Co.,Ltd.
Xiamen AmoyChem Co., Ltd
Hubei xin bonus chemical co. LTD
Shandong chuangyingchemical Co., Ltd.
Chongqing Chemdad Co., Ltd





