|
|
| | tert-Butyl thiophen-2-ylcarbamate Basic information | | Structure |
| Product Name: | tert-Butyl thiophen-2-ylcarbamate | | Synonyms: | TERT-BUTYL N-(2-THIENYL)CARBAMATE;TERT-BUTYL 2-THIENYLCARBAMATE;THIOPHEN-2-YL-CARBAMIC ACID TERT-BUTYL ESTER;BUTTPARK 97\57-48;AKOS 92405;tert-Butyl thiophen-2-ylcarbaMate;CarbaMic acid, N-2-thienyl-, 1,1-diMethylethyl ester;tert-Butyl thien-2-ylcarbamate | | CAS: | 56267-50-6 | | MF: | C9H13NO2S | | MW: | 199.27 | | EINECS: | | | Product Categories: | | | Mol File: | 56267-50-6.mol | 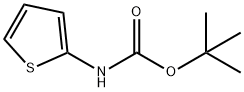 |
| | tert-Butyl thiophen-2-ylcarbamate Chemical Properties |
| Melting point | 151 °C | | Boiling point | 238.1±13.0 °C(Predicted) | | density | 1.186±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C(protect from light) | | form | solid | | pka | 13.63±0.70(Predicted) | | color | Pale grey | | InChI | InChI=1S/C9H13NO2S/c1-9(2,3)12-8(11)10-7-5-4-6-13-7/h4-6H,1-3H3,(H,10,11) | | InChIKey | QTXXTRMGTVEBIN-UHFFFAOYSA-N | | SMILES | C(OC(C)(C)C)(=O)NC1SC=CC=1 |
| Hazard Codes | Xn | | Risk Statements | 22 | | HS Code | 2934999090 |
| | tert-Butyl thiophen-2-ylcarbamate Usage And Synthesis |
| Structure |
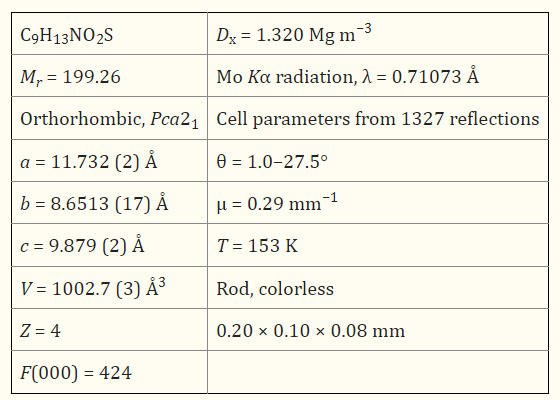
Tert-butyl thiophen-2-ylcarbamate, C9H13NO2S, is a precursor in synthesising diimine ligands suitable for metal complex formation. This compound exhibits intramolecular methyl C7—H···O1 and C8—H···O1 interactions [2.938 (4) and 3.109 (4), respectively] in addition to bulky tert -butyl groups. These two features in tandem allow the packing in the crystal to be nearly perpendicular [the angle between adjacent thiophene rings = 74.83 (7)°]. An intermolecular N1—H···O1i hydrogen bond gives a one-dimensional chain which extends along [0 0 1].
| | Chemical Properties | White to off-white crystalline powder | | Synthesis | To a solution of thiophenecarboxylic acid (0.5 g, 3.90 mmol) in tBuOH (10 ml) was added Et3N (0.571 ml, 4.10 mmol) and DPPA (0.883 ml, 4.10 mmol). The solution was heated at 90 °C for 4 hours. The reaction mixture was cooled to RT, and the solvent was removed from the vacuum. The residue was treated with benzene, and the solution was washed with 5 per cent citric acid and NaHCO3. Solid was filtered off, and the filtrate was washed with brine. The organic layer was dried (MgSO4), concentrated in vacuo and the residue was purified by silica gel column chromatography (EtOAc/hexanes) to obtain tert-butyl thiophen-2-ylcarbamate (0.39 g, 50percent yield). 1H NMR (400 MHz, DMSO-d6): δ 10.4 (brs, 1H), 6.84 (dd, J = 1.6, and 5.2 Hz, 1H), 6.75 (dd, J = 4.0, and 5.6 Hz, 1H), 6.48 (dd, J= 1.6, and 4.0 Hz, 2H), 1.45 (s, 9H); MS (ESI) m/z: 222.0 (M+22+H+). |
| | tert-Butyl thiophen-2-ylcarbamate Preparation Products And Raw materials |
|