| | Phenobarbital Chemical Properties |
| Melting point | 174°C | | Boiling point | 374.4°C (rough estimate) | | density | 1.2243 (rough estimate) | | refractive index | 1.6660 (estimate) | | Fp | 11 °C | | storage temp. | 2-8°C | | solubility | Very slightly soluble in water, freely soluble in ethanol (96 per cent). It forms water-soluble compounds with alkali hydroxides, carbonates and ammonia. | | form | Solid | | pka | 7.3, 11.8(at 25℃) | | color | White to Off-White | | Water Solubility | <0.01 g/100 mL at 14 ºC | | Merck | 13,7319 | | BCS Class | 1 | | Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. | | CAS DataBase Reference | 50-06-6(CAS DataBase Reference) | | IARC | 2B (Vol. Sup 7, 79) 2001 | | NIST Chemistry Reference | Barbituric acid, 5-ethyl-5-phenyl-,(50-06-6) | | EPA Substance Registry System | Phenobarbital (50-06-6) |
| | Phenobarbital Usage And Synthesis |
| Antiepileptic | Phenobarbital, also known as Rumina and Canaa, is white andglossy crystalline powder. Phenobarbital is a non- proprietary first- generation antiepileptic drug. Exposed to air at normal temperature, it is stable in nature, difficult to dissolve in water and insoluble in acid. It can be dissolved in ethanol, ether, acetone and other organic solvents. This product has sedative, hypnotic and anticonvulsant effects, and can resist epilepsy. It is effective for large epileptic seizures, localized seizures and status epilepticus. It also enhances the role of antipyretic analgesics, and can induce liver microsomal glucuronosyltransferase activity, promote bilirubin and glucuronic acid binding, reduce plasma bilirubin concentration, and treat neonatal cerebral nucleus jaundice.
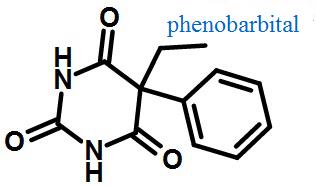
Figure 1 Structural formula of phenobarbital | | Barbiturate drugs | Barbiturate: It is a type of tranquilizer that acts on the central nervous system and is a derivative of barbituric acid. Its application ranges from mild sedation to complete anaesthesia, and can also be used as antianxiety drugs, sleeping pills, and antispasmodic drugs. Long term use leads to addiction.
| | Physicochemical properties | Physical properties: barbiturates are usually white crystal or crystalline powder. Stable in the air. General slightly soluble or soluble in water, soluble in ethanol, ethyl ether, dissolved in chloroform.
Sodium salt is easy to dissolve in water and is difficult to dissolve in organic solvent.
Chemical properties: a. Faintly acidic: in the hexatomic ring of barbiturates, the 1,3- imidodicarbonic diamide group can occur ketone enol tautomerism and ionize in aqueous solution. Therefore, barbiturate is weak acidity, and pKa is 7.3 to 8.4.
b.Easy hydrolysis: the imidodicarbonic diamide group can be hydrolyzed under the alkaline condition and release ammonia gas.
c. Reaction with heavy metal ions:If the silver nitrate is added to the Barbital sodium carbonate solution,it will generate white insoluble disilver salt; With pyridine and copper sulfate solution, it will generate blue violet complex for identification and measurement of content. The action mechanism of Barbiturate drug.Barbiturates act on the synaptic transmission process of the netting excitatory system, block the network structure and activate the system, reduce the excitability of the cortical cells, resulting in stabilization, hypnotic and anticonvulsant effects. This effect is mainly seen in the synapses of GABA neurotransmitters. It enhances the GABA mediated Cl- inflow and weakens the depolarization mediated by glutamate. Barbiturates increase the Cl- internal flow by prolonging the opening time of the chloride channel, causing hyperpolarization. At higher concentrations, it inhibits the Ca2+ dependent action potential, inhibits the release of Ca2+ dependent transmitters, and presents a quasi GABA effect. That is, it can also increase the Cl- inflow directly when there is no GABA.
| | Pharmacological action | This product is a sedative hypnotic and anticonvulsant and it is atypical representative of long-acting barbiturate. The inhibitory effect on the central nervous system increases with the dose. It is characterized by sedative, hypnotic, anticonvulsant and antiepileptic.
Large doses have obvious inhibition on the cardiovascular system and respiratory system.Excessive use can paralyze the medulla of the respiratory center and thus cause death. In the electrophysiological experiment in vitro, it is found that phenobarbital makes the chloride channels of the nerve cells open and the cells are polarised, which is similar to the role of gamma aminobutyric acid (GABA).The treatment concentration of phenobarbital can reduce the excitatory effect of glutamate and strengthen the inhibitory effect of gamma aminobutyric acid, It inhibits the transmission of single synapses and multiple synapses in the central nervous system, and inhibits the high frequency discharge of the epileptic focus and its diffusion around the central nervous system.
| | Pharmacokinetics | After oral administration, it is absorbed completely but slowly in the digestive tract. It will take effect 0.5 to 1 hours after injection, and the concentration of blood drug reaches the peak value after 2~18 hours. After absorption, the plasma protein binding rate is about 40% (20% ~ 45%), and the apparent volume is 0.5 ~ 0.9L/kg.The concentration is highest in the brain tissue. The largest amount of medicine is in the skeletal muscle. It can pass through the placenta. Its effective blood concentration is about 10~40 g/ml, and it will have toxic reaction when the blood concentration is more than 40μg/ml. The adult half-life (t1/2) is about 50~144 hours, the child is about 40~70 hours, and the half-life (t1/2) will be prolonged if the liver and kidney function worse. About 48% ~ 65% of phenobarbital is metabolized in the liver and converted to phenobarbital. This product is a liver enzyme inducer to improve the activity of the enzyme, not only to accelerate the metabolism of its own, but also to accelerate the metabolism of other drugs.Most of this product is combined with glucuronic acid or sulfate, excreted by the kidneys.There are 27% to 50% of the prototypes excreted from the kidneys.It can pass through the placenta and secrete human milk.
| | Indications | It is mainly used for the treatment of anxiety, insomnia (used for short sleep time, early awakening), epilepsy and dyskinesia.
It is an important drug for the treatment of major epileptic seizures and localized seizures.It can also be used as a drug for anti hyperbilirubinemia and before anesthesia.Injection is used for the treatment of epilepsy. It is effective for systemic and partial seizures. It is generally used when phenytoin, carbamazepine and valproate are ineffective. It can also be used for other diseases caused by convulsions and before anesthesia.
| | Adverse reaction |
- The most common adverse reaction for antiepileptic seizures is sedation. But as the course continues, its sedative effect gradually becomes unobvious.
- It may cause subtle emotional changes, and a defect in cognition and memory.In the long-term medication, occasionally folic acid deficiency and hypocalcemia can be seen.
- Megaloblastic anemia and osteomalacia are rarely seen.
- In large doses, nystagmus, ataxia, and severe respiratory depression can be produced.1% to 3% of the patients using this product have skin reactions.The most common sights are rashes. Exfoliative dermatitis and polymorphous erythema or Stevens-Johnson syndrome may occur in severe cases, and toxic epidermal necrosis is extremely rare.
- There are reports of hepatitis and liver dysfunction.
- Drug dependence may occur for a long time drug taking.It is easy to withdrawal syndrome after stopping drug use.
| | Precaution |
- People allergic to barbiturate may also be allergic to this product.For antiepileptic drug application, it may take 10~30 days to reach the maximum effect. It is necessary to calculate the amount of medicine by weight. If possible, it is necessary to determine the concentration of blood drug regularly to reach the maximum effect.
- In patients suffer from liver dysfunction, the dosage should start from a small amount.Long-term use of drugs can produce mental or physical dependence on drugs. The withdrawal of drugs should be gradually reduced, so as to avoid the withdrawal symptoms.
- Combined with other central suppressor drugs, it has a synergistic inhibitory effect on the center, and should be paid attention to.Be cautious in the following cases: minimal brain dysfunction, hypotension, hypertension, anemia, hypothyroidism and adrenal insufficiency, liver and kidney function damage, aerial, driver, fine and dangerous type operators.
- This medicine may pass through the placenta,Long term use in pregnancy can cause dependence and drug withdrawal syndrome.
- Neonatal hemorrhage may be caused by reduced vitamin K content.If it is used in the late pregnancy or childbirth, because the fetal liver function is not mature, it can cause the respiratory depression of the newborn (especially the premature infant).It may have a teratogenic effect on the fetus. FDA classifies it as grade D for the safety of pregnancy. The application of it during lactation may cause the suppression of the central nervous system in the baby.
- It may cause abnormal excitement, and attention should be paid to it.
- The usual dose of this drug can cause excitement, insanity or depression. Therefore, the dosage should be small.
| | Drug interaction | 1. This product is a liver enzyme inducer, which can improve the activity of the enzyme. Long term use not only accelerates its metabolism, but also accelerates the metabolism of other drugs. Before using anaesthesia, such as halothane, enflurane, methoxane etc., if the barbiturate has been taken for a long time, it can increase the metabolites of the anesthetic and increase the risk of liver toxicity. When barbiturates are used simultaneously with ketamine, especially large doses of intravenous administration, it can increase the risk of lowering blood pressure and breathing inhibition.
2. When combined with oral anticoagulants, it can reduce the effect of the latter.
3. Its combined use with oral contraceptives can reduce the reliability of the contraceptive. Its combination use with estrogen and estrogen reduces the effect of estrogen.It can reduce the effects of these drugs when it is combined with corticosteroids, digitalis (including digoxin), oxytetracycline, or tricyclic antidepressants.
4.In combination with cyclophosphamide, it can theoretically increase the alkylation of cyclophosphamide metabolites, but the clinical significance is not clear.
5. When combined with quinidine, it attenuates the effect of quinidineby increasing the metabolism of quinidine.It is combined with a calcium channel blocker, causing a drop in blood pressure. When combined with fluperbutanol for the treatment of epilepsy, it can cause changes in the form of epileptic seizures and doses need to be adjusted.
6. When combined with phenothiazine and tetracyclic antidepressants, it can reduce the seizure threshold and increase the inhibitory effect. Combined with ibuprofen it can reduce or shorten the half-life and reduce the intensity of action.
| | Interactions | With AEDs
- Both primidone and its major metabolite phenobarbital are metabolized by, and also induce, liver enzyme activity (especially the CYP 450 3A4 enzyme system). There are a number of interactions which are potentially clinically significant.
- Phenobarbital and primidone plasma concentrations are increased by oxcarbazepine, phenytoin and valproate.
- Vigabatrin possibly decreases phenobarbital and primidone plasma concentrations.
- Phenobarbital and primidone therapy may also lead to altered pharmacokinetics in concomitantly administered AEDs, whose metabolism may be increased, and lead to lowered plasma levels and/ or a shorter halflife: carbamazepine, ethosuximide, lamotrigine, oxcarbazepine, phenytoin, valproate, tiagabine, topiramate, zonisamide.
With other drugs
- Agents which inhibit the CYP 450 3A4 enzyme system, such as chloramphenicol, nelfinavir, and metronidazole may result in increased plasma levels of concomitantly administered primidone and its metabolite phenobarbital.
- In addition, St John’s Wort induces the CYP450 enzyme system and may result in a reduction of plasma levels of concomitantly administered primidone and of its metabolite phenobarbital.
- Phenobarbital and primidone therapy may also lead to altered pharmacokinetics in concomitantly administered drugs, whose metabolism may be increased, and lead to lowered plasma levels and/ or a shorter half- life. These drugs include androgens, beta- antagonists, ciclosporin, clozapine, chloramphenicol, corticosteroids/ glucocorticosteroids, cyclophosphamide, dicoumarins, digitoxin, doxycycline, etoposide, granisetron, losartan, methadone, metronidazole, mianserin, montelukast, nelfinavir, nimodipine, oral contraceptives, quinidine, rocuronium, theophyllines, tricyclic antidepressants, vecuronium, and warfarin.
- The central nervous system (CNS) depressant effect of phenobarbital and primidone is additive to those of other CNS depressants such as opiates.
With alcohol/food
Concurrent administration with alcohol may lead to an additive CNS depressant effect and there are no specific foods that must be excluded from diet when taking phenobarbital or primidone.
| | Special populations | Hepatic impairment
Reduce dose as it may precipitate coma (avoid in severe impairment).
Renal impairment
Use with caution.
Pregnancy
- Phenobarbital and primidone therapy in pregnant women with epilepsy present a risk to the foetus in terms of major and minor congenital defects, such as congenital craniofacial, heart, and digital abnormalities, as well as cleft lip and palate.
- In case of treatment during pregnancy, the dose of phenobarbital and primidone should be monitored carefully and adjustments made on a clinical basis.
- Phenobarbital and primidone readily cross the placenta following oral administration and are distributed throughout foetal tissue, the highest concentrations being found in the placenta, foetal liver and brain. Adverse effects on neurobehavioural development and withdrawal symptoms have been reported in the newly born whose mothers have received phenobarbital or primidone during late pregnancy
- Phenobarbital and primidone are excreted into breastmilk and there is a small risk of neonatal sedation. During breastfeeding, the baby should be monitored for sedation, although breastfeeding is not advisable.
| | Behavioural and cognitive effects in patients with epilepsy | Patients taking phenobarbital have been shown to have a high prevalence of major depressive disorder and suicidal ideation. A long history of exposure to barbiturates may carry the greatest risk of depression, particularly in patients taking polytherapy and patients with a personal or family history of affective disorders. Similarly to benzodiazepines, barbiturates can also induce a paradoxycal syndrome characterized by insomnia, hyperactivity, impulsiveness and aggressiveness (especially in patients with learning disability). Barbiturates are more frequently associated with adverse cognitive side effects than most other AEDs. The spectrum of cognitive problems reported by patients with epilepsy taking phenobarbital encompasses attention, memory, and language.
| | Psychiatric use | Barbiturates have no approved indications in psychiatry. Off-label uses have previously included sedative-hypnotic withdrawal and alcohol- withdrawal (as alternative to benzodiazepines).
| | Description | Phenobarbital (Item No. 9001494) is an analytical reference material categorized as a barbiturate. Phenobarbital is regulated as a Schedule IV compound in the United States. This product is intended for research and forensic applications. | | Chemical Properties | Crystalline Solid | | Originator | Phenobarbital ,Inter-Chemical Ltd. | | Uses | This is a controlled substance (depressant). Anticonvulsant; sedative; hypnotic | | Uses | Phenobarbital exhibits relaxant, soporific, and anticonvulsant activities. It is widely used
in treating epilepsy, chorea, and spastic paralysis, and is used as a component of a large
number of combined drugs, valocordin and corvalol in particular. | | Indications | Phenobarbital can reduce cholestatic pruritus, possibly
by enhancing hepatic microsomal function. Phenobarbital is sedating and
may interfere with the metabolism of many drugs. | | Definition | ChEBI: A member of the class of barbiturates, the structure of which is that of barbituric acid substituted at C-5 by ethyl and phenyl groups. | | Manufacturing Process | 528 g phenylethyl malonic diethyl ester is dissolved in 500 ml of absolute alcohol. There is then added 140 g urea to the mixture. To this mixture is then added a solution of 57.5 g sodium in 1000 ml absolute alcohol, at such rate that one-half the solution is added during the first hour, a quarter the second hour; an eighth the third hour, and the final eighth during the 4 hours. Then the alcohol is distilled from the reaction mixture. When the alcohol has all been removed, 250 ml xylol is added to the mixture. The reaction mixture is cooled to room temperature and 3 L of water added. The xylene layer was separated and the water solution washed with another 200 ml portion of xylene There is then added to the water solution a 10% excess of a 50% by weight solution of sulfuric acid. The phenobarbital is precipitated as nearly white fluffy crystals, which are filtered off. When dried, they showed 100% phenobarbital by titration. This product may be purified by recrystallization. The unreacted ester in the xylene solution was recovered by distilling off the xylene, and then the phenylethyl malonic ester. | | Brand name | Luminal Sodium (Sterling Winthrop);3p spas;Aaciasthma;Adocor;Allergasthmin;Anaspaz;Anti-spas;Asmo fedrilum;Asthmatussin;Bakersed;Barbellen;Barberine;Barbilletae;Barcole;Barophen;Bay-ase;Bebtoyl;Bediphen;Belergamin;Bellademal s;Belladenal;Bellasectal;Bellastal;Bellergal s;Belllumal;Bergofen;Bock-ase;Bonexyl;Broncosmin;C 147;Ce 10010;Ceepa;Cemealonal;Clemodril;Coffecodin;Cor-asthmolyticum;Cortasmyl;Corverum;Dafodil;Digi-pulsnorma;Dithene-r;Dolo-eupaco;Donibin;Donna-lix;Donnaplex;Eeskabarb span;Elibese;Elmigrin;Ephedrobarbital-t;Ephestmin;Epidormb;Ergojuvan;Espafren;Extrovent;Fasconal;Fedrinal;Fenalgin;Fenilcal;Fenosed bitabs;Gardenale;Gastrop;Giolate;Glyanphen;Glyuferal;Gourmase;Gratusminal;Hasp;Hyonol;Ila-med;Irs 109a;Kenedes;Koronar;Lagaspasm;Lardet;Legatin;Lircapil;Lumcalcio;Lunadon;Lysadestal;Mazur-a;Md 1020;Mediphen;Meprobit;Mepropon;Metrojen;Mialgone;Migrane-dolviran;Modirit;Neo-nervostat;Nilspasm;Novodon;Oxabar;Oxoids;Pavadel;Peba;Pen-nitate;Phen bar;Phen-bel;Phenogen;Pheno-gesic;Phental;Phentral cratecil;Piraminal;Plivalgin;Preminal;Prenoxan;Pribetal;Purphen;Quad-sed;Rau-fridetten;Resirol;Respisane;S 611-3;Salviton;Sapos;Scotatal;Secophen-c;Sedacoral;Seda-intestain;Seda-ko;Sedalgin;Sedapar;Sedo corodil;Sedopsic;Sedragesic;Solofoton;Soniphen;Spascol;Spasdel;Spasmalones;Spasmo-compragyl;Spasmogentarol;Spasmotal;Spasmo-van;Spasmoveragin;Spastyl;Spondyneuron;Stollerine;Supamidal;Susano;Syntospon;Tedralan;Teofedrin;Thefedral;Theodrine;Theotabs;Vanital;Zirkonorm. | | Therapeutic Function | Anticonvulsant, Antiepileptic, Hypnotic, Sedative | | World Health Organization (WHO) | Phenobarbital is a long-acting barbiturate which is controlled
under Schedule IV of the 1971 Convention on Psychotropic Substances.
Phenobarbital is of value in the treatment of epilepsy and preparations for such
use are included in the WHO Model List of Essential Drugs. See also WHO
comment for barbiturates.
(Reference: (UNCPS4) United Nations Convention on Psychotropic Substances (IV),
, , 1971) | | General Description | Odorless white crystalline powder or colorless crystals. A saturated aqueous solution is acid to litmus (approximately pH 5). Slightly bitter taste. | | General Description | Phenobarbital, 5-ethyl-5-phenylbarbituricacid (Luminal), is a long-acting sedative and hypnotic.It is also a valuable anticonvulsant, especially in generalizedtonic–clonic and partial seizures (see the discussionon anticonvulsants). Metabolism to the p-hydroxylphenylcompound followed by glucuronidation accounts for about90% of a dose. | | Air & Water Reactions | Sensitive to hydrolysis. Alkaline solutions react more rapidly than acidic solutions. At pH 7 and 176°F, has a half life of 74 hours. Insoluble in water. | | Reactivity Profile | Phenobarbital is also sensitive to prolonged exposure to light. Incompatible with strong oxidizing agents. Forms a complex of reduced solubility with macrogol 4000. Able to form metal derivatives . | | Fire Hazard | Phenobarbital is combustible. | | Clinical Use | Antiepileptic | | Safety Profile | Confirmed carcinogen
with experimental carcinogenic,
neoplastigenic, tumorigenic, and teratogenic
data. A human poison by ingestion. An
experimental poison by ingestion,
intraperitoneal, subcutaneous, intravenous,
and rectal routes. Human systemic effects by
ingestion: somnolence, motor activity
changes, pulmonary changes, allergc
dermatitis, and fever. Human reproductive
effects by ingestion: drug dependence and
other postnatal measures or effects. Human
teratogenic effects include developmental abnormalities of the central nervous system,
body wall, musculoskeletal, respiratory,
gastrointestinal, and urogenital systems.
Experimental reproductive effects. Human
mutation data reported. Used as a drug in
the treatment of epilepsy, and as a hypnotic
and sedative. When heated to
decomposition it emits toxic fumes of NOx.See also BARBITURATES. | | Synthesis | Phenobarbital, 5-ethyl-5-phenylbarbituric acid or 5-ethyl-5-phenylhexa�hydropyrimindin-2,4,6-trione (4.1.4), has been synthesized in several different ways [1¨C4].
There is no major difference between them. The first method consists of ethanolysis
of benzyl cyanide in the presence of acid, giving phenylacetic acid ethyl ether, the
methylene group of which undergoes acylation using the diethyloxalate, giving diethyl
ester of phenyloxobutandioic acid (4.1.1), which upon heating easily loses carbon oxide
and turns into phenylmalonic ester (4.1.2). Alkylation of the obtained product using
ethylbromide in the presence of sodium ethoxide leads to the formation of |á-phenyl-|á-
ethylmalonic ester (4.1.3), the condensation of which with urea gives phenobarbital
(4.1.4) [1]. 
Another method of phenobarbital synthesis starts with condensation of benzyl cyanide with
diethylcarbonate in the presence of sodium ethoxide to give |á-phenylcyanoacetic ester
(4.1.5). Alkylation of the ester (4.1.5) using ethylbromide gives |á-phenyl-|á-ethylcyanoacetic ester (4.1.6), which is further converted into the 4-iminoderivative (4.1.7). Acidic hydrolysis
of the resulting product gives phenobarbital (4.1.4) [2].
| | Drug interactions | Potentially hazardous interactions with other drugs
Aminophylline and theophylline: metabolism of
aminophylline and theophylline increased, reduced
effect.
Anthelmintics: concentration of albendazole and
praziquantel reduced.
Anti-arrhythmics: reduced concentration of
disopyramide; possibly reduced concentration of
dronedarone - avoid; possibly increases metabolism
of propafenone.
Antibacterials: reduced concentration of
chloramphenicol, doxycycline, metronidazole,
telithromycin and rifampicin - avoid with
telithromycin.
Anticoagulants: increased metabolism of coumarins
(reduced effect); concentration of apixaban, edoxaban
and rivaroxaban reduced.
Antidepressants: antagonise anticonvulsant effect;
reduces concentration of paroxetine, reboxetine,
mianserin and tricyclics; concentration reduced by St
John’s wort - avoid.
Antiepileptics: concentration increased by
oxcarbazepine, phenytoin, stripentol and valproate
and possibly carbamazepine, also active metabolite of
oxcarbazepine reduced and valproate concentration
reduced, concentration of fosphenytoin and
phenytoin usually reduced but can also be increased;
concentration of ethosuximide, rufinamide and
topiramate possibly reduced; concentration of
lamotrigine, tiagabine and zonisamide reduced.
Antifungals: possibly reduced concentration of
itraconazole, isavuconazole, posaconazole and
voriconazole - avoid with voriconazole; reduced
absorption of griseofulvin (reduced effect).
Antimalarials: avoid with piperaquine with
artenimol; anticonvulsant effect antagonised by
mefloquine.
Antimuscarinics: possibly reduces active metabolite
of fesoterodine - avoid.
Antipsychotics: antagonise anticonvulsant effect;
metabolism of haloperidol increased; possibly
reduces aripiprazole concentration - increase
aripiprazole dose; concentration of both drugs
reduced with chlorpromazine; possibly reduces
clozapine concentration; possibly reduces lurasidone
concentration - avoid.
Antivirals: concentration of abacavir, boceprevir,
darunavir, fosamprenavir, indinavir, lopinavir,
rilpivirine and saquinavir possibly reduced; avoid
with boceprevir and rilpivirine; possibly reduces
daclatasvir, dasabuvir, ombitasvir, paritaprevir
and simeprevir concentration - avoid; avoid with
elvitegravir, etravirine, ledipasvir, sofosbuvir and
telaprevir; possibly reduces concentration of
dolutegravir.
Apremilast: possibly reduces concentration of
apremilast - avoid.
Bile acids: avoid with cholic acid.
Calcium-channel blockers: effects of calcium-channel
blockers probably reduced - avoid with isradipine
and nimodipine.
Cannabis extract: possibly reduces concentration of
cannabis extract - avoid.
Ciclosporin: reduced ciclosporin levels.
Cobicistat: possibly reduces concentration of
cobicistat - avoid.
Corticosteroids: metabolism of corticosteroids
accelerated, reduced effect.
Cytotoxics: possibly reduced concentration
of axitinib, increase axitinib dose; possibly
reduced concentration of bortezomib, bosutinib,
cabozantinib, ceritinib, crizotinib, dasatinib,
ponatinib and vandetanib - avoid; avoid with
cabazitaxel, ceritinib, dabrafenib, gefitinib, olaparib
and panobinostat; concentration of irinotecan and
its active metabolite and possibly etoposide reduced;
possible increased hypersensitivity reactions with
procarbazine.
Diuretics: concentration of eplerenone reduced -
avoid; increased risk of osteomalacia with carbonic
anhydrase inhibitors.
Guanfacine: concentration of guanfacine possibly
reduced - increase dose of guanfacine.
Hormone antagonists: possibly reduced
concentration of abiraterone - avoid; metabolism of
toremifene accelerated.
Ivacaftor: possibly reduced concentration of ivacaftor
- avoid.
Oestrogens and progestogens: metabolism
accelerated, reduced contraceptive effect.
Orlistat: possibly increased risk of convulsions.
Sodium oxybate: enhanced effects of sodium oxybate
- avoid.
Tacrolimus: concentration of tacrolimus reduced.
Ulipristal: contraceptive effect reduced - avoid. | | Metabolism | Partly metabolised in the liver.
25% of a dose is excreted in the urine unchanged at
normal urinary pH. |
| | Phenobarbital Preparation Products And Raw materials |
|