- Afatinib impurity J
-
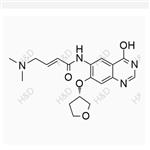
- $0.00 / 10mg
-
2024-09-20
- CAS:
- Min. Order: 10mg
- Purity: 98%
- Supply Ability: 500mg
- Afatinib impurity 57
-
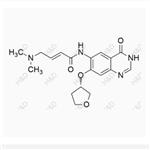
- $0.00 / 10mg
-
2024-09-20
- CAS:1456696-14-2
- Min. Order: 10mg
- Purity: 98%
- Supply Ability: 500mg
|
| | Afatinib IMpurity J Basic information |
| Product Name: | Afatinib IMpurity J | | Synonyms: | Afatinib IMpurity J;Afatinib Impurity J HCl;(2E)-N-[3,4-Dihydro-4-oxo-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-2-butenamide;Afatinib impurity 8/(2E)-N-[3,4-Dihydro-4-oxo-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-2-butenamide;(2E)-4-(Dimethylamino)-N-{4-oxo-7-[(3S)-tetrahydro-3-furanyloxy]-1,4-dihydro-6-quinazolinyl}-2-butenamide;2-Butenamide, N-[3,4-dihydro-4-oxo-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-, (2E)-;(S,E)-4-(dimethylamino)-N-(4-oxo-7-((tetrahydrofuran-3-yl)oxy)-3,4-dihydroquinazolin-6-yl)but-2-enamide;Afatinib B | | CAS: | 1456696-14-2 | | MF: | C18H22N4O4 | | MW: | 358.39 | | EINECS: | 604-604-1 | | Product Categories: | | | Mol File: | 1456696-14-2.mol | 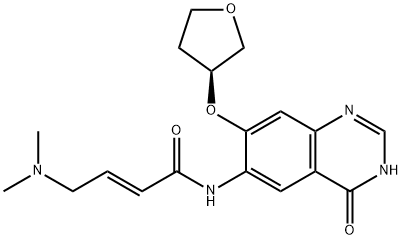 |
| | Afatinib IMpurity J Chemical Properties |
| density | 1.33±0.1 g/cm3(Predicted) | | pka | 11.89±0.20(Predicted) |
| | Afatinib IMpurity J Usage And Synthesis |
| Uses | (2E)-N-[3,4-Dihydro-4-oxo-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-2-butenamide, s an impurity of Afatinib (A355300), an aminocrotonylamino-substituted quinazoline derivative used for treating cancer and diseases of the respiratory tract, lungs, gastrointestinal tract, bile duct, and gallbladder. |
| | Afatinib IMpurity J Preparation Products And Raw materials |
|