|
|
| | COBALT CARBONATE Basic information |
| Product Name: | COBALT CARBONATE | | Synonyms: | COBALTOUS CARBONATE;COBALT (II) CARBONATE;COBALT(II) CARBONATE, BASIC;Carbonic acid/cobalt,(1:x) salt;Carbonic acid, cobaltsalt (1:?) | | CAS: | 7542-09-8 | | MF: | CCoO3 | | MW: | 118.94 | | EINECS: | | | Product Categories: | Inorganics | | Mol File: | 7542-09-8.mol | 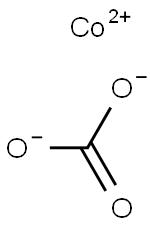 |
| | COBALT CARBONATE Chemical Properties |
| Melting point | decomposes [HAW93] | | solubility | insoluble in H2O; soluble in acid solutions | | color | red-violet crystals, crystalline | | Water Solubility | insoluble cold H2O, decomposed by hot H2O; soluble acids [HAW93] | | EPA Substance Registry System | Cobalt carbonate (7542-09-8) |
| Provider | Language |
|
ALFA
| English |
| | COBALT CARBONATE Usage And Synthesis |
| Chemical Properties | This occurs naturally as sphaerocobaltite. It can be precipitated
as the violet-red hexahydrate CoCO3. 6H2O from aqueous solutions of cobalt(II) salts and
an alkali metal bicarbonate if the reaction is carried out under an atmosphere of carbon
dioxide. In the absence of carbon dioxide basic salts are precipitated in these reactions.
The hydrate is dehydrated to the CoCO3 at 140° and at 350° in vacuo CoO is formed. | | Chemical Properties | Red crystals.
Insoluble in water and ammonia; soluble in acids.
The cobalt carbonate of commerce is usually the
basic salt (see following entry). | | Uses | Ceramics, trace element added to soils and animal feed, temperature indicator, catalyst, pigments. |
| | COBALT CARBONATE Preparation Products And Raw materials |
|