- Montelukast
-

- $6.00 / 1kg
-
2024-12-17
- CAS:158966-92-8
- Min. Order: 1kg
- Purity: 95%
- Supply Ability: 1000kg
- Montelukast
-

- $0.00 / 1KG
-
2024-12-13
- CAS:158966-92-8
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 100kg
- Montelukast
-
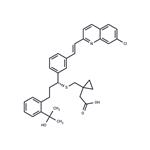
- $0.00 / 50mg
-
2024-11-19
- CAS:158966-92-8
- Min. Order:
- Purity: 99.74%
- Supply Ability: 10g
|
| | Montelukast Basic information |
| Product Name: | Montelukast | | Synonyms: | Montelukast D6 sodium salt (Rac);Cyclopropaneaceticacid,1-[[[(1R)-1-[3-[(1E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-Methylethyl)phenyl]propyl]thio]Methyl]-;Montelukast(Form A);Montelukast Acid;Montelukast;Montelukas;(r-(e))-1-(((1-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-(2-(1-hydroxy-1-methylethyl)phenyl)propyl)thio)methyl)cyclopropaneacetic acid;Montelukastl | | CAS: | 158966-92-8 | | MF: | C35H36ClNO3S | | MW: | 586.18 | | EINECS: | 256-723-9 | | Product Categories: | 158966-92-8 | | Mol File: | 158966-92-8.mol | 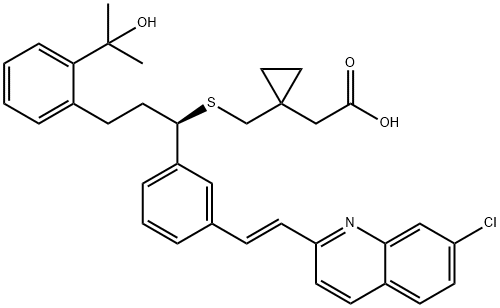 |
| | Montelukast Chemical Properties |
| | Montelukast Usage And Synthesis |
| Originator | Singulair,Merck Pharmaceutical,Canada | | Uses | cardiostimulant, | | Definition | ChEBI: Montelukast is a member of quinolines, a monocarboxylic acid and an aliphatic sulfide. It has a role as a leukotriene antagonist, an anti-asthmatic drug and an anti-arrhythmia drug. It is a conjugate acid of a montelukast(1-). | | Manufacturing Process | Crotonaldehyde (3.23 mol) in 100 mL of 2-butanol was added dropwise to a
refluxing solution of 4-chloroaniline (3.23 mol), p-chloranil (3.23 mol) and HCl
conc. (808 mL) in 5.4 L of 2-butanol. After 2 hours of heating 2.7 L of solvent
was removed under vacuum at 60°C. Then 2 L of toluene was added to the
reaction mixture followed by removal of 2.5-3 L of solvent until a very pasty
solid formed. THF (2 L) was added and the mixture heated 30 min after which
it was cooled to 0°C. The solid was collected and washed with THF until pure
by tlc. The solid was then dissolved in aq. K2CO3/EtOAc and the organic phase
separated. The aqueous phase was extracted with EtOAc and the organic
phases combined, dried over MgSO4 and the solvent removed. The product
was crystallized in the minimum amount of EtOAc to give 328.08 g (57%) of
4-chloro-2-methylquinolin.
4-Chloro-2-methylquinalin was converted into 3-(2-(7-chloro)-2-
quinolinyl)ethenyl)benzaldehyde. Reaction was carried out according to a
method described in U.S. Pat. No. 4,851,409
To a degassed suspension of 3-(2-(7-chloro-2-quinolinyl)ethenyl)benzaldehyde
(0.34 mol) in toluene (700 mL) at 0°C was added 1.0 M vinylmagnesium
bromide in toluene/THF (370 mL). After stirring for 1 hour at 0°C, the
reaction was quenched by the addition of saturated NH4Cl solution (150 ml),
followed by H2O (500 mL) and HOAc (50 mL). The product was extracted with
EtOAc and the two-phase system was filtered through celite to remove an
insoluble precipitate. The aqueous phase was then re-extracted with EtOAc
(100 mL) and the combined organic layer was washed with H2O, followed by
brine. The solution was dried (MgSO4), and evaporated to give a dark yellow
residue which was purified by flash chromatography (EtOAc:hexane 1:5, then
1:3). The product was filtered from the column fractions to give a solid of 1-
(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-2-propen-1-ol (melting point =
110-112°C). The filtrate was concentrated and the resulting residue was
recrystallized from EtOAc/hexane 1:4 to give a second crop of 15.1 g.
A degassed suspension of 1-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-2-propen-1-ol (46.6 mmol), n-Bu4NCl (93 mmol), LiOAcH2O (115 mmol), LiCl
(93 mmol), Pd(OAc)2 (1.4 mmol) and methyl 2-(2-iodophenyl)propanoate in
DMF (90 mL) was stirred for 2 hours at 100°C. The dark red solution was then
cooled to 0°C and poured into saturated NaHCO3 solution (500 mL). The
product was extracted with EtOAc and the organic layer was washed with H2O
followed by brine. The solvent was removed under vacuum and the residue
was purified by flash chromatography (EtOAc:hexane 1:10, 1:5 and 3:10) to
give a pale yellow foam of ethyl 2-(3(S)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)
phenyl)-3-hydroxy-propyl)benzoate (18.9 g).
A mixture of anhydrous CeCl3 (164 mmol) in THF (500 mL) was refluxed
overnight using a Dean Stark trap filled with activated molecular sieves.
Methyl magnesium chloride (3.0 Molar solution in THF, 790 mmol) was added
dropwise over 30 min to the CeCl3 slurry at 0°C. After stirring 2 hours, the
mixture was cooled to -5°C and a toluene (600 mL) solution of the ethyl 2-
(3(S)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-hydroxy-propyl)benzoate
(152 mmol) was added dropwise over 1 hour. The reaction mixture was stirred
another hour before the addition of 2 M HOAc (600 mL) and toluene (600
mL). The organic layer was washed with saturated aq. NaHCO3 and with brine.
Concentration in vacuo and purification of the residue by flash
chromatography (30% EtOAc in toluene) gave 63.48 g (91%) of the 2-(2-
(3(S)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-hydroxypropyl)phenyl)-2-
propanol.
To a solution of BH3THF complex (1 M in THF, 262 mL) was added diethyl 1,1-
cyclopropanedicarboxylate (134 mmol) at 25°C under N2. The solution was
heated at reflux for 6 hours, cooled to r.t., and MeOH (300 mL) was cautiously
added. The solution was stirred for 1 hour and then concentrated to an oil.
The crude 2-(2-(3(S)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-
hydroxypropyl)phenyl)-2-propanol was dissolved in CH2Cl2 (234 mL) and
SOCl2 (15.9 g, 134 mmol) was added dropwise over a period of 15 min at
25°C. After stirring for another 15 min, the mixture was washed with aqueous
NaHCO3. The organic extract was dried over Na2SO4, filtered and concentrated
to give quantitatively the 1,1-cyclopropanedimethanol cyclic sulfite.
To a solution of the 1,1-cyclopropanedimethanol cyclic sulfite (99 mmol) in
DMF (83 mL) was added NaCN (199 mmol). The mixture was heated to 90°C
for 20 hours. Upon cooling, EtOAc (400 mL) was added and the solution was
washed with saturated NaHCO3 solution (55 mL), H2O (4 times 55 mL),
saturated NaCl solution and dried over Na2SO4. The solution was concentrated
to give 7.1 g (65%) of 1-(hydroxymethyl)cyclopropaneacetonitrile.
To a solution of 1-(hydroxymethyl)cyclopropaneacetonitrile (42 g, 378 mmol)
in dry CH2Cl2 (450 mL) at -30°C was added Et3N (741 mmol) followed by
CH3SO2Cl (562 mmol) dropwise. The mixture was warmed to 25°C, washed
with NaHCO3, dried over Na2SO4 and concentrated in vacuo to give the
corresponding mesylate. The mesylate was then dissolved in DMF (450 mL)
and cooled to 0°C. Potassium thioacetate (55.4 g, 485 mmol) was added, and
the mixture was stirred at 25°C for 18 hours. EtOAc (1.5 L) was added, the
solution was washed with NaHCO3, dried over Na2SO4 and concentrated in
vacuo to give 45 g (70%) of 1-(acetythiomethyl)cyclopropaneacetonitrile.
To a solution of the 1-(acetythiomethyl)cyclopropaneacetonitrile (266 mmol)
in MeOH (1.36 L) was added H2O (84 mL) and conc. H2SO4(168 mL). The
mixture was heated to reflux for 20 hours, cooled to 25°C, H2O (1 L) was
added and the product was extracted with CH2Cl2. The organic extract was
washed with H2O and dried over Na2SO4. Concentration of the organic solution
gave 36 g (93%) of the methyl 1-(thiomethyl)cyclopropaneacetate.
To a solution of 2-(2-(3(S)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-
hydroxypropyl)phenyl)-2-propanol in THF was dissolved in THF (1 mL) and
DMF (1 mL) at -40°C was added diisopropylethylamine (2.2 mmol) and then
methanesulfonyl chloride (2.2 mmol). The mixture was stirred 2 hours with
slow warming to -30°C. The methyl 1-(thiomethyl)cyclopropaneacetate (2.3
mmol) was added to the cloudy reaction mixture followed by dropwise
addition of potassium tert-butoxide/THF solution (4.4 mmol). The reaction
mixture was stirred at -30°C for 3.5 hours before quenching it with 25% aq
NH4OAc. Extraction with EtOAc, washing the organic layer with brine and
evaporation of the solvents left a residue that was purified by flash
chromatography (5%-10% EtOAc in toluene) giving 658 mg (53%) of methyl
1-((((R)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-(2-(2-hydroxy-2-
propyl)phenyl)propyl)thio)methyl)cyclopropaneacetate.
Following the hydrolysis the methyl 1-((((R)-(3-(2-(7-chloro-2-quinolinyl)
ethenyl)phenyl)-3-(2-(2-hydroxy-2-propyl)phenyl)propyl)thio)methyl)
cyclopropaneacetate with NaOH was obtained the free acid: 4-((1(R)-(3-(2-(7-
chloro-2-quinolinyl)ethenyl)phenyl)-3-(2-(2-hydroxy-2-propyl)-phenyl)propyl)
thio)methyl)cyclopropaneacetic acid or sodium 1-(((1(R)-(3-(2-(7-chloro-2-
quinolinyl)ethenyl)phenyl)-3-(2-(2-hydroxy-2-propyl)phenyl)propyl)thio)
methyl) cyclopropaneacetate. | | Brand name | Singulair (Merck). | | Therapeutic Function | Anti-asthmatic | | Mechanism of action | Montelukast was developed from other weakly antagonistic quinoline derivatives. A number of changes can be made to the structure without the loss of activity. These include changing the double bond between the two aromatic rings to an ether linkage, reducing the quinoline ring, changing the chlorine to a fluorine, and/or exchanging the sulfur for an amide group. | | Pharmacokinetics | Montelukast is a high-affinity, selective antagonist of the cysLT1 receptor. It is rapidly absorbed orally, with a bioavailability of 64%. Montelukast is 99% bound to plasma proteins and is extensively metabolized in the liver by CYP3A4 and CYP2C9 to oxidated products. CYP3A4 oxidizes the sulfur and the C-21 benzylic carbon, whereas CYP2C9 is selectively responsible for the methyl hydroxylation. | | Clinical Use | Leukotriene receptor antagonist:
Prophylaxis of asthma
Seasonal allergic rhinitis | | Side effects | Montelukast did not demonstrate any significant adverse effects greater than placebo in clinical trials; however, because it is metabolized by the cytochrome P450 (CYP450) enzymes, its plasma levels should be monitored when coadministered with CYP450-inducing drugs, such as phenobarbital, rifampin, and phenytoin. | | Metabolism | Extensively metabolised in the liver by cytochrome P450
isoenzymes CYP3A4, CYP2A6, and CYP2C9.
Excreted principally in the faeces via the bile. Metabolites
have minimal therapeutic activity. |
| | Montelukast Preparation Products And Raw materials |
|