| Company Name: |
J & K SCIENTIFIC LTD.
|
| Tel: |
010-82848833 400-666-7788 |
| Email: |
jkinfo@jkchemical.com |
| Products Intro: |
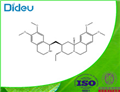
Product Name:EMetine (>90%)
CAS:483-18-1
Package:10Mg,1Mg
|
- EMETINE USP/EP/BP
-
- $1.10 / 1g
-
2021-06-18
- CAS:483-18-1
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons Min
|
| | Emetine Basic information |
| Product Name: | Emetine | | Synonyms: | EMETINE;(2S,3R,11bS)-3-Ethyl-1,3,4,6,7,11b-hexahydro-9,10-diMethoxy-2-[[(1R)-1,2,3,4-tetrahydro-6,7-diMethoxy-1-isoquinolinyl]Methyl]-2H-benzo[a]quinolizine.;EMetin;Emetine HCL BP/USP;6',7',10,11-Tetramethoxymetan;Cephaeline methyl ether;Canforemetina��、Emetine Hydrochloride;Emetine (base and/or unspecified salts) | | CAS: | 483-18-1 | | MF: | C29H40N2O4 | | MW: | 480.64 | | EINECS: | 207-592-1 | | Product Categories: | Chiral Reagents;Impurities;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 483-18-1.mol | 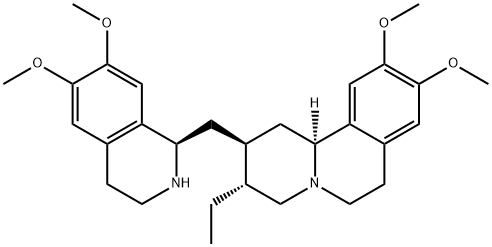 |
| | Emetine Chemical Properties |
| Melting point | 89-96°C | | alpha | D20 -50° (c = 2 in CHCl3) | | Boiling point | 578.63°C (rough estimate) | | density | 1.1174 (rough estimate) | | refractive index | 1.5800 (estimate) | | storage temp. | -20°C Freezer | | solubility | Soluble in Water (100 mg/ml) | | form | solid | | pka | 5.77, 6.64(at 25℃) | | color | White | | Water Solubility | 961.3mg/L(15 ºC) | | Stability: | Stable for 2 years from date of purchase as supplied. Solutions in water may be stored at -20°C for up to 3 months. |
| | Emetine Usage And Synthesis |
| Description | Emetine hydrochloride (483-18-1) is one of the active ingredients of ipecac root extract, used as an emetic.1 Induces apoptosis in breast cancer cells via inhibition of Wnt/β-catenin signaling.2 Inhibits Zika and Ebola virus in vitro and in vivo, targeting viral entry and replication by inhibiting viral RNA polymerase and host lysosomal function.3 Also inhibits SARS-CoV-2 replication in cells (EC50 for viral load reduction is 0.46 μM).4 A useful agent for inhibiting protein synthesis in eukaryotic cells by virtue of its inhibition of the ribosome 40S subunit.5 | | Chemical Properties | Brown Solid | | Uses | Emetine is the principal alkaloid of ipecac, the ground roots of Uragoga ipecacuanha. | | Uses | Emetine occurs in the ground roots ofipecac (Uragoga ipecacuanha, Cephaelisipecacuanha, Uragoga acuminata, and Rubiaceae). Ipecacs are of two varieties, Brazilianand Cartagena. The former contains about1.2–1.5% emetine, and the latter constitutesabout 1.1–1.4% emetine. It is used as anemetic to induce vomiting for the treatmentof poisoning. It is also used as an antiamebic. | | Indications | Chlorprothixene (Taractan) has been reported twice to be effective in the
treatment of PHN. For severe pain, an initial 50 to 100 mg IM injection has
been advocated. Otherwise, the dosage is 25 to 50 mg PO q6h. The recommended
duration of therapy is 4 to 10 days. Higher doses are unwarranted and
frequently result in side effects. | | Definition | ChEBI: Emetine is a pyridoisoquinoline comprising emetam having methoxy substituents at the 6'-, 7'-, 10- and 11-positions. It is an antiprotozoal agent and emetic. It inhibits SARS-CoV2, Zika and Ebola virus replication and displays antimalarial, antineoplastic and antiamoebic properties. It has a role as an antiprotozoal drug, a plant metabolite, an antiviral agent, an emetic, a protein synthesis inhibitor, an antimalarial, an antineoplastic agent, an autophagy inhibitor, an antiinfective agent, an expectorant, an anticoronaviral agent and an antiamoebic agent. It is a pyridoisoquinoline and an isoquinoline alkaloid. It is functionally related to a cephaeline. It is a conjugate base of an emetine(2+). It derives from a hydride of an emetan. | | Brand name | Asmorex;Broncho-tetracycline;Dicton-retard;Emedrin;Emetocamphrol;Hemometina;Optairosol;Pectinfant. | | World Health Organization (WHO) | Emetine, an alkaloid obtained from ipecacuanha, was first used
rationally as an amoebocide in 1912. It was subsequently widely used and was
included in earlier editions of the WHO Model List of Essential Drugs but has now
been replaced by the less cardiotoxic synthetic derivative dehydroemetine.
Although it is valuable in the treatment of systemic amoebic hepatitis it has now
been largely superseded by considerably less toxic drugs, and in particular by
metronidazole. | | Hazard | Toxic by ingestion. | | Health Hazard | The toxicity of cephaeline is lower than thatof emetine. The toxic effects are cumulative. Ingestion of high doses may producehypotension, muscle weakness, and gastroin testinal problems, including nausea, vomit ing, and diarrhea. | | Mechanism of action | This drug has a direct amebicidal effect against trophozoites E. histolytica in tissues, and
it is not active against cysts in either the lumen or intestinal walls, or in other organs.
The mechanism of action of emetine consists of the blockage of protein synthesis in
eukaryotic (but not in prokaryotic) cells. It inhibits the process of polypeptide chain
formation. Protein synthesis is inhibited in parasite and mammalian cells, but not in
bacteria.
Emetine is currently only used as a drug for treating amebiasis in cases of resistance to
other drugs. Synonyms of this drug are ipecin and methylcefalin. | | Synthesis | Emetine, 3-ethyl-2,3,4,6,7,11b-hexahydro-9,10-dimethoxy-2-(1,2,3,4-tetrahydro-
6,7-dimethoxy-1-isoquinolylmethyl)-1H-benzo[a]quinolizine (37.2.8), is a natural com�pound. Traditionally, the alkaloid emetine was extracted from the ipecacuanha plant
(Brazilian root) and used as the primary drug for treating amebiasis, leishaniasis, and dysen�tery. Various ways of synthesizing emetine have been suggested, all of which begin with
homoveratrylamine ¨C 2-(3,4-dimethoxyphenyl)ethylamine.
Upon a combined catalytic hydrogenation of the ethyl ester of |?¨C(|á??-cyano)propylglu�taric acid and homoveratrilamine, a reductive amination reaction takes place, in which
ammonia is released and an intermediate amine (37.2.5) is formed, which under the reac�tion conditions undergoes intramolecular cyclization to give 1-[2-(3,4-dimethoxyphenyl)-
ethyl]-4-carbethoxymethyl-6-ethylpiperidone-2 (37.2.6). Reacting the resulting lactam
with phosphorus oxychloride causes heterocyclization into the derivative of benzoquino�lizine (37.2.7). Subsequent reaction of the product with homoveratrylamine makes the cor�responding amide. Upon reaction with phosphorus oxychloride, this compound cyclizes to
an isoquinoline derivative, and the pyridine ring is then hydrogenated by hydrogen to a
racemic mixture of products, from which the desired emetine is isolated.
| | References | 1) Lee et al. (2008), Ipecacuanha: the South American vomiting root; J R Coll. Physicians Edinb., 38 355
2) Sun et al. (2019), Emetine Exhibits Anticancer Activity in Breast Cancer Cells as an Antagonist of Wnt/β-catenin Signaling; Oncol. Rep., 42 1735 DOI:10.3892/or.2019.7290
3) Yang et al. (2018), Changing cancer survival in China during 2003-2015: a pooled analysis of 17 population-based cancer registries; Cell Discov., 4 31
4) Choy et al. (2020), Remdesivir, Lopinavir, and Homoharringtonine Inhibit SARS-CoV-2 Replication in Vitro; Antivir. Res., 178 104786 DOI:10.1016/j.antiviral.2020.104786
5) Cuyas et al. (2015), Anti-protozoal and Anti-Bacterial Antibiotics That Inhibit Protein Synthesis Kill Cancer Subtypes Enriched for Stem Cell-Like Properties; Cell Cycle, 14 3527
DOI:10.1080/15384101.2015.1044173
6) Application of emetine in SARS-CoV-2 treatment: regulation of p38 MAPK signaling pathway for preventing emetine-induced cardiac complications DOI:10.1080/15384101.2022.2100575
7) Emetine, a potent alkaloid for the treatment of SARS-CoV-2 targeting papain-like protease and non-structural proteins: pharmacokinetics, molecular docking and dynamic studies DOI:10.1080/07391102.2021.1946715
8) A Comprehensive Guide to the Hazardous Properties of Chemical Substances
9) Manual of Dermatologic Therapeutics with Essentials of Diagnosis, 7th Edition
10) Consolidated List of Products whose Consumption |
| | Emetine Preparation Products And Raw materials |
|