|
|
| | 1-(2,2-diMethoxyethyl)-5-Methoxy-6-(Methoxycarbonyl)-4-oxo-1,4-dihydropyridine-3-carboxylic acid Basic information |
| Product Name: | 1-(2,2-diMethoxyethyl)-5-Methoxy-6-(Methoxycarbonyl)-4-oxo-1,4-dihydropyridine-3-carboxylic acid | | Synonyms: | 1-(2,2-Dimethoxyethyl)-1,4-dihydro-3-methoxy-4-oxo-2,5-pyridinedicarboxylic acid 2-methyl ester;1-[2,2-bis(methyloxy)ethyl]-5-(methyloxy)-6-[(methyloxy)carbonyl]-4-oxo-1,4-dihydro-3-pyridinecarboxylic acid;1-(2,2-diMethoxyethyl)-5-Methoxy-6-(Methoxycarbonyl)-4-oxo-1,4-dihydropyridine-3-carboxylic acid;Dulotegravir Me-N-4;2,5-Pyridinedicarboxylic acid, 1-(2,2-dimethoxyethyl)-1,4-dihydro-3-methoxy-4-oxo-, 2-methyl ester;Dolutegravir Intermediates3;1-(2,2-Dimethoxyethyl)-1,4-dihydro-3-methoxy-4-oxo-2,5-pyridinedicarboxylic acid 2-methyl este;1-(2,2-dimethoxyethyl)-5-methoxy-4-oxo-1,4-dihydropyridine-3,6-dicarboxylic acid-6-methyl ester | | CAS: | 1335210-23-5 | | MF: | C13H17NO8 | | MW: | 315.28 | | EINECS: | | | Product Categories: | CMLLYL | | Mol File: | 1335210-23-5.mol | 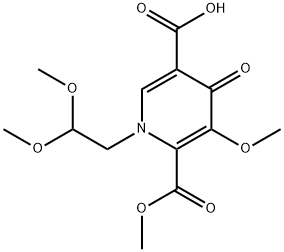 |
| | 1-(2,2-diMethoxyethyl)-5-Methoxy-6-(Methoxycarbonyl)-4-oxo-1,4-dihydropyridine-3-carboxylic acid Chemical Properties |
| Boiling point | 474.1±45.0 °C(Predicted) | | density | 1.36±0.1 g/cm3(Predicted) | | storage temp. | Inert atmosphere,Room Temperature | | solubility | Chloroform, DMSO (Slightly), Methanol (Slightly) | | pka | 5.56±0.40(Predicted) | | form | Solid | | color | White to Light Yellow |
| | 1-(2,2-diMethoxyethyl)-5-Methoxy-6-(Methoxycarbonyl)-4-oxo-1,4-dihydropyridine-3-carboxylic acid Usage And Synthesis |
| Uses |
Dolutegravir intermediate-1 (1-(2,2-diMethoxyethyl)-5-Methoxy-6-(Methoxycarbonyl)-4-oxo-1,4-dihydropyridine-3-carboxylic acid) is used as an intermediate in the synthesis of GSK1265744 (I), a potent HIV integrase inhibitor.
| | Synthesis |
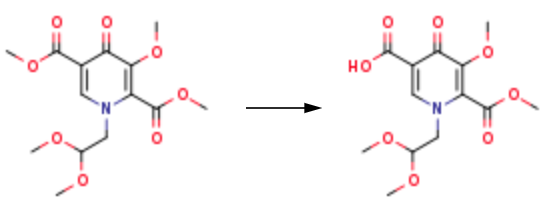
N, N-Dimethylformamide dimethyl acetal was added to methyl 4-methoxy-3-oxobutanoate, and the mixture was kept for 2 h at 25-30°C. The resulting methyl2-(dimethylaminomethylidene)-4-methoxy-3-oxobutanoate was dissolved in methanol, 2-amino acetaldehyde dimethyl acetal was added, and the mixture was stirred at20-30°C for 2 h. The solvent was removed under reduced pressure, the residue was diluted with methanol, and dimethyl oxalate was added at 25-30°C. The mixture was cooled to 0-5°C, 30 wt % sodium methoxide in methanol was slowly added, and the mixture was slowly heated to 40°C, stirred for 14 h at that temperature, and concentrated under reduced pressure. The residue was diluted with dichloromethane and poured into cold (0-5°C)2 N aqueous HCl. The organic phase was separated, the aqueous layer was extracted with dichloromethane (2× ), and the combined organic layers were washed with demineralized water and concentrated under reduced pressure at 40-45 °C to obtain intermediate. The product was dissolved in methanol, the solution was cooled to 0-5°C, and lithium hydroxide monohydrate was added. The mixture was stirred at 0-5°C for 3 h, quenched with 2 N aqueous HCl, and extracted with ethyl acetate (3×). Finally, the product 1-(2,2-diMethoxyethyl)-5-Methoxy-6-(Methoxycarbonyl)-4-oxo-1,4-dihydropyridine-3-carboxylic acid can be obtained through further purification.
|
| | 1-(2,2-diMethoxyethyl)-5-Methoxy-6-(Methoxycarbonyl)-4-oxo-1,4-dihydropyridine-3-carboxylic acid Preparation Products And Raw materials |
| Raw materials | methyl 2-(((2,2-dimethoxyethyl)amino)methylene)-4-methoxy-3-oxobutanoate-->Dimethyl oxalate | | Preparation Products | (2R,5S,13aR)-8-methoxy-7,9-dioxo-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxylic acid-->(4R,12aS)-7-Methoxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxylic acid |
|