- Chlorpromazine
-

- $66.00 / 1kg
-
2024-01-18
- CAS:50-53-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000
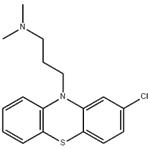
- Chlorpromazine
-
- $0.00 / 5mg
-
2023-11-01
- CAS:50-53-3
- Min. Order: 1mg
- Purity: 98% HPLC
- Supply Ability: 100kg
Related articles - Uses of Chlorpromazine
- Chlorpromazine is a first-generation (‘typical’) antipsychotic approved by the US Food and Drug Administration (FDA) in 1957.
- Jan 10,2022
|
| | Chlorpromazine Basic information |
| Product Name: | Chlorpromazine | | Synonyms: | Novomazina;Phenactyl;Phenathyl;Phenothiazine, 2-chloro-10-[3-(dimethylamino)propyl]-;Plegomasine;Prazil;Prazilpromactil;Propaphenin | | CAS: | 50-53-3 | | MF: | C17H19ClN2S | | MW: | 318.86 | | EINECS: | 200-045-8 | | Product Categories: | THORAZINE | | Mol File: | 50-53-3.mol | 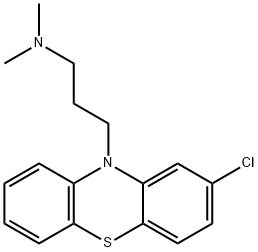 |
| | Chlorpromazine Chemical Properties |
| Melting point | 56.5°C | | Boiling point | bp0.8 200-205° | | density | 1.1644 (rough estimate) | | refractive index | 1.6230 (estimate) | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Solid | | pka | pKa 9.3(H2O,t =24±1) (Uncertain) | | color | White to Off-White | | NIST Chemistry Reference | Chlorpromazine(50-53-3) | | EPA Substance Registry System | Chlorpromazine (50-53-3) |
| | Chlorpromazine Usage And Synthesis |
| Description | This phenothiazine with sedative properties is used in
human medicines and has induced contact dermatitis
in nurses or in those working in the pharmaceutical
industry. It is also used in veterinary medicine to avoid
mortality of pigs during transportation. It is a sensitizer
and a photosensitizer. | | Chemical Properties | Oily liquid; amine odor. | | Uses | antiemetic, antipsychotic | | Uses | In psychiatric practice, chlorpromazine is used in various conditions of psychomotor
excitement in patients with schizophrenia, chronic paranoid and also manic-depressive
conditions, neurosis, alcohol psychosis and neurosis accompanied by excitement, fear,
stress, and insomnia. In comparison with other neuroleptics, chlorpromazine is unique in
that it has an expressed sedative effect. It is sometimes used in anesthesiological practice
for potentiating narcosis. It also has moderate anticonvulsant action. | | Uses | Chlorpromazine is approved by FDA for use in humans for the
management of psychotic disorders (i.e., control of mania,
treatment of schizophrenia); control of nausea and vomiting;
relief of apprehension before surgery; acute intermittent
porphyria; adjunctive treatment of tetanus; intractable hiccups;
combativeness or explosive hyperexcitable behavior in children
aged 1–12 years; and short-term treatment of hyperactivity in
children with symptoms of impulsivity, difficulty sustaining
attention, aggressiveness, mood lability, and poor frustration
tolerance. Chlorpromazine is commonly used off-label for
treatment of behavioral symptoms associated with dementia in
the elderly and psychosis and agitation related to Alzheimer’s
dementia; however, it carries a boxed warning regarding increased risk of death in patients with dementia-related
psychosis. Chlorpromazine is also used off-label for managing
agitation in terminal cancer patients, autonomic dysreflexia,
cancer pain, adjunctive treatment of cholera, migraine headaches,
opioid withdrawal, ocular pain, paralytic ileus, and
phantom limb syndrome.
In veterinary medicine, the use of chlorpromazine has been
largely replaced by the phenothiazine acepromazine due to its
more favorable pharmacokinetic profile. Chlorpromazine may
be used as an antiemetic for small animals or for preoperative
sedation. Chlorpromazine may also be used for management
of hypertension in dogs and cats. | | Definition | ChEBI: A substituted phenothiazine in which the ring nitrogen at position 10 is attached to C-3 of an N,N-dimethylpropanamine moiety. | | Brand name | Thorazine (GlaxoSmithKline). | | Hazard | Toxic by ingestion. | | Contact allergens | This phenothiazine with sedative properties is used in
human medicine and induced contact dermatitis in nurses
or those working in the pharmaceutical industry. It is also
used in veterinary medicine to avoid mortality of pigs during
transportation. It is a sensitizer and a photosensitizer. | | Synthesis | Chlorpromazine, 2-chloro-10-(3-dimethylaminopropyl)phenothiazine, is synthesized in an analogous manner, except by alkylation of 2-chlorophenoth�iazine with 3-dimethylaminopropylchloride. | | Environmental Fate | Acute and chronic toxicity due to chlorpromazine generally
manifests as an extension of normal pharmacological activity.
The precise mechanism of action of chlorpromazine, and other
phenothiazines, is unknown; however, it is thought to primarily
involve antagonism of dopaminergic (D2) neurotransmission
at synaptic sites and blockade of postsynaptic dopamine
receptor sites at the subcortical levels of the reticular formation,
limbic system, and hypothalamus. This activity contributes to
chlorpromazine’s extrapyramidal reactions. Chlorpromazine
also has strong central and peripheral activity directed against
adrenergic receptors and weak activity against serotonergic,
histaminic (H1), and muscarinic receptors. Chlorpromazine
has slight ganglionic blocking action. Chlorpromazine is
known to depress vasomotor reflexes medicated by the hypothalamus
and/or brain stem; inhibit release of growth hormone;
antagonize secretion of prolactin release-inhibiting hormone;
and reduce secretion of corticotropin-regulatory hormone.
Chlorpromazine also has direct effects on cardiac myocytes;
it can induce early after-depolarizations, block depolarizing
sodium channels, and cause significant prolongation of the
QTc interval.
Chlorpromazine may be irritating to eyes, mucous
membranes, and skin. Contact and inhalation should be
avoided. | | Metabolic pathway | The in vivo photodegradation of chlorpromazine in rat
skin exposed to UV-A results in the formation of
promazine and 2-hydroxypromazine in irradiated rats,
but not in the skin of rats kept in the dark.
Chlorpromazine sulfoxide is a major metabolite of
chlorpromazine, found in smaller quantity in the skin of
irradiated rats compared with those kept in the dark.
Chlorpromazine sulfoxide is not a photoproduct of
chlorpromazine under the experimental conditions. | | Toxicity evaluation | Chlorpromazine exists as both a vapor and particulate at
ambient atmospheric conditions. Chlorpromazine vapor is
degraded by photochemically produced hydroxyl radicals
with an estimated half-life of 1.6 h. Chlorpromazine particulate
is removed by wet or dry deposition. Chlorpromazine is
likely to be immobile in soil (Koc 9900, pKa 9.3) and to
adsorb to sediment if released into water. It is not expected to
volatilize from soil or water. There is high potential for
bioconcentration. |
| | Chlorpromazine Preparation Products And Raw materials |
|