- Malonamide
-

- $0.00 / 25KG
-
2025-03-21
- CAS:108-13-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Malonamide
-

- $10.00 / 1kg
-
2025-03-20
- CAS:108-13-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 mt
- Malonamide
-

- $9.00 / 1KG
-
2024-10-11
- CAS:108-13-4
- Min. Order: 1KG
- Purity: 99.8%
- Supply Ability: 100tons
|
| | Malonamide Basic information |
| | Malonamide Chemical Properties |
| Melting point | 172-175 °C (lit.) | | Boiling point | 191.38°C (rough estimate) | | density | 1.3516 (rough estimate) | | refractive index | 1.5110 (estimate) | | storage temp. | Sealed in dry,Room Temperature | | solubility | Soluble in HCl. | | pka | 7.00±0.70(Predicted) | | form | powder to crystal | | color | White to Almost white | | Water Solubility | 180 g/L (20 ºC) | | BRN | 1751401 | | InChI | InChI=1S/C3H6N2O2/c4-2(6)1-3(5)7/h1H2,(H2,4,6)(H2,5,7) | | InChIKey | WRIRWRKPLXCTFD-UHFFFAOYSA-N | | SMILES | C(N)(=O)CC(N)=O | | CAS DataBase Reference | 108-13-4(CAS DataBase Reference) | | NIST Chemistry Reference | Propanediamide(108-13-4) | | EPA Substance Registry System | Propanediamide (108-13-4) |
| Safety Statements | 22-24/25 | | WGK Germany | 2 | | RTECS | ON9125000 | | TSCA | Yes | | HS Code | 29241900 |
| | Malonamide Usage And Synthesis |
| Description | Malonamide is a dicarboxylic acid diamide that is malonic acid in which both carboxy groups have been replaced by carbamoyl groups. It is functionally related to a malonic acid. | | Chemical Properties | White crystalline powder | | Uses | Malonamide is a reagent for fluorimetric determination of reducing carbohydrates. The malonamide-based ionic liquid extractant was used in the extraction of europium(iii) and other trivalent rare earth elements from nitric acid medium. | | Uses | The malonamide-based ionic liquid extractant was used in the
extraction of europium(iii) and other trivalent rare-earth ions from
nitric acid medium. | | Uses | Malonamide is used in the synthesis of malonamic acid, malonamate and malonamide derivative of some heterocyclic compounds with antiinflammatory activity. | | Definition | ChEBI: Malonamide is a dicarboxylic acid diamide that is malonic acid in which both carboxy groups have been replaced by carbamoyl groups. It is functionally related to a malonic acid. | | General Description | The malonamide derivatives are obtained by the one-pot, five-component condensation reaction of isocyanide, Meldrum′s acid, arylidene malononitrile, and two amine molecules in CH2Cl2. | | Hazard | Mildly toxic by ingestion. | | Synthesis | The synthesis of Malonamide is as follows:
A typical reaction was carried out in a 10 mL flask. Benzonitrile (2 mmol), CuII-4 ? (0.2 g), acetaldoxime (6 mmol) and MeOH (4 mL) were stirred at 65 °C for 4 h. The solid was filtered, washed with MeOH and the filtrate evaporated. The residue was subjected to GC-MS analysis and NMR spectroscopy. The filtered catalyst can be recycled after drying at about 150 °C for 1 h.
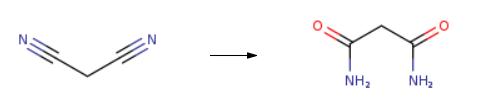 | | Purification Methods | Crystallise the amide from water. [Beilstein 2 IV 1887.] |
| | Malonamide Preparation Products And Raw materials |
|