- Nitrocefin
-
- $0.00 / 1g
-
2024-11-18
- CAS:41906-86-9
- Min. Order: 1g
- Purity: 90%
- Supply Ability: 100g
- Nitrocefin
-
- $66.00 / 1mg
-
2024-11-07
- CAS:41906-86-9
- Min. Order:
- Purity: ≥98%
- Supply Ability: 10g
- NITROCEFIN
-

- $0.00 / 1kg
-
2023-06-30
- CAS:41906-86-9
- Min. Order: 1kg
- Purity: NA
- Supply Ability: 5000kgs
|
| | Nitrocefin Basic information |
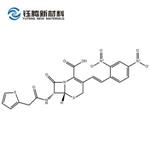
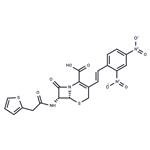
| Product Name: | Nitrocefin | | Synonyms: | 3-(2,4-DINITROSTYRYL)-(6R, 7R)-7-(2-THIENYLACETAMIDO)-CEPH-3-EM-4 CARBOXYLIC ACID, E-ISOMER;(6R)-3-[(E)-2-(2,4-Dinitrophenyl)ethenyl]-8-oxo-7α-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;Nitrocefin (>90%);(6R,7R)-3-[(E)-2-(2,4-dinitrophenyl)ethenyl]-8-oxo-7-[(2-thiophen-2-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;NITROCEFIN;5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[(1E)-2-(2,4-dinitrophenyl)ethenyl]-8-oxo-7-[(2-thienylacetyl)amino]-, (6R,7R)-;Nitrocefin (>;3-[(E)-2-(2,4-Dinitrophenyl)vinyl]-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | | CAS: | 41906-86-9 | | MF: | C21H16N4O8S2 | | MW: | 516.5 | | EINECS: | | | Product Categories: | APIs | | Mol File: | 41906-86-9.mol | 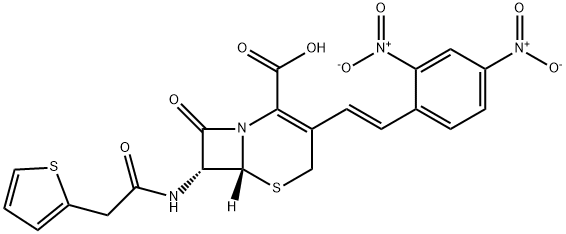 |
| | Nitrocefin Chemical Properties |
| Melting point | 103-113° (dec); mp 167-169° (dec) (Lee) | | alpha | D20 -224° (c = 1.0 in dioxane) | | Boiling point | 872.0±65.0 °C(Predicted) | | density | 1.67±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | DMF: 20 mg/ml; DMSO: 20 mg/ml; DMSO:PBS (pH 7.2) (1:20): 0.04 mg/ml | | form | A crystalline solid | | pka | 2.50±0.50(Predicted) | | color | Yellow to orange | | Stability: | Hygroscopic, Unstable in Solution | | InChIKey | LHNIIDJCEODSHA-OQRUQETBSA-N | | SMILES | N12[C@@]([H])([C@H](NC(CC3SC=CC=3)=O)C1=O)SCC(/C=C/C1=CC=C([N+]([O-])=O)C=C1[N+]([O-])=O)=C2C(O)=O |
| | Nitrocefin Usage And Synthesis |
| Description | The generation of β-lactamases by bacteria affords resistance to several classes of β-lactam antibiotics, including penicillins and cephalosporins. Nitrocefin is a chromogenic cephalosporin substrate commonly used to detect β-lactamases in bacteria. The presence of β-lactamase activity is indicated by the appearance of a red color that is proportional in intensity to the original concentration of nitrocefin. | | Chemical Properties | Nitrocefin is the chromogenic cephalosporin that acts as an excellent β-lactamase substrate. It exhibits a rapid distinctive color change from yellow (max at pH 7.0 = 390 nm) to red (max at pH 7.0 = 486 nm) as the amide bond in the beta-lactam ring is hydrolyzed by a β-lactamase. It is sensitive to hydrolysis by all known lactamases produced by Gram-positive and Gram-negative bacteria.
Nitrocefin (Yellow) --β-lactamase-->Product (Red) (OD486nm)
Solution preparation and color change before and after β-lactamase exposure

(A) Concentrated nitrocefin (10.0 mg/mL) in DMSO before dilution with PBS buffer. (B) Nitrocefin diluted with PBS buffer
to working concentration (1.0 mg/mL). The yellow color is indicative of intact, undegraded nitrocefin. (C) 25 units of betalactamase dropped on top of nitrocefin (1.0 mg/mL in PBS). The red color is the result of beta-lactamase mediated
cleavage of the nitrocefin. (D) Vortexed mixture of contents shown in picture (C). | | Uses | Nitrocefin is a chromogenic β-lactamase substrate that undergoes colour change from yellow to red as the amide bond in the β-Lactam ring is hydrolyzed by β-lactamase. Nitrocefin undergoes colour chang
es induced by lactamases produced by Gram-positive and Gram-negative bacteria. Several studies have utilized the colour changing properties of Nitrocefin for the detection of β-lactamase activity from
bacterial cell extracts by isoelectric focusing and spectroscopy. Nitrocefin has also been used in studies involving β-lactamase resistant antibiotics. | | Preparation | Nitrocefin is a key reagent for high and low throughput assays of the activities of penicillin-binding proteins (PBPs) and β-lactamases, the former used for discovery of antibiotics and the latter for inhibitors of resistance determinants for β-lactam antibiotics. This compound is commercially available but is prohibitively expensive because of the circuitous routes to its synthesis. We describe herein a three-step synthesis of nitrocefin that gives an overall yield of 44%. This is a practical route to the synthesis of this key reagent for drug discovery.
A Practical Synthesis of Nitrocefin | | Biological Functions | In determination of b-lactamase activity in biological samples.
Nitrocefin is a colorless or faint yellow cephalosporin antimicrobial that is hydrolyzed rapidly by most beta-lactamases.The hydrolysis product is pink.The bacterium to be tested is applied to a paper disk containing nitrocefin. A pink color developing within minutes (positive test) indicates a beta-lactamaseproducing bacterium. |
| | Nitrocefin Preparation Products And Raw materials |
|









