- Cianidanol
-

- $6.00 / 1kg
-
2024-12-20
- CAS:154-23-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month
- Cianidanol
-

- $6.00 / 1kg
-
2024-12-19
- CAS:154-23-4
- Min. Order: 1kg
- Purity: More than 99%
- Supply Ability: 2000KG/Month
|
| | Cianidanol Basic information |
| Product Name: | Cianidanol | | Synonyms: | (+)-CATECHIN;CATECHIN, (+)-;(+)-3,3'4',5,7-FLAVANEPENTOL;3,3',4',5,7-PENTAHYDROXYFLAVANE;[2R,3S]-2-[3,4-DIHYDROXYPHENYL]-3,4-DIHYDRO-1[2H]-BENZOPYRAN-3,4,7-TRIOL;2-(3,4-dihydroxyphenyl)-3,4-dihydro-(2r-trans)-2h-1-benzopyran-7-triol;5,7-triol,2-(3,4-dihydroxyphenyl)-3,4-dihydro-,(2R-trans)-2H-1-Benzopyran-3;catechin(flavan) | | CAS: | 154-23-4 | | MF: | C15H14O6 | | MW: | 290.27 | | EINECS: | 205-825-1 | | Product Categories: | Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;Pharmaceutical Intermediates;Biochemistry;Flavonoids;Natural Plant Extract;Aromatics;Chiral Reagents;Elisa Kit-plant ELISA Kit | | Mol File: | 154-23-4.mol | 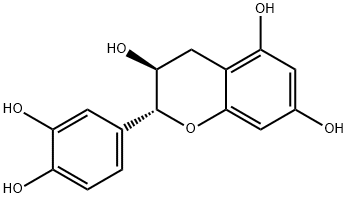 |
| | Cianidanol Chemical Properties |
| Melting point | 175-177 °C (anhydrous)(lit.) | | Boiling point | 352.36°C (rough estimate) | | alpha | D18 +16 to +18.4° | | density | 1.2451 (rough estimate) | | refractive index | 1.4359 (estimate) | | storage temp. | 2-8°C | | solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly, Sonicated, Heated) | | form | Solid | | pka | 9.54±0.10(Predicted) | | color | Light Orange to Light Brown | | Merck | 14,1902 | | InChIKey | PFTAWBLQPZVEMU-DZGCQCFKSA-N | | LogP | 0.510 | | CAS DataBase Reference | 154-23-4 | | EPA Substance Registry System | 2H-1-Benzopyran-3,5,7-triol, 2-(3,4-dihydroxyphenyl)-3,4-dihydro-, (2R,3S)- (154-23-4) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | RTECS | DJ3450000 | | F | 8-10-23 | | HS Code | 29329990 | | Toxicity | LD50 oral in rat: > 10gm/kg |
| | Cianidanol Usage And Synthesis |
| Chemical Properties | Tan Solid | | Uses | Labelled (+)-Catechin (C217500). (+)-Catechin is a flavonoid found primarily in higher woody plants as (+)-Catechin along with (-)-Epicatechin (cis form).
Antidiarrheal. | | Uses | procollagen production inhibitoe, hepatoprotectant | | Uses | Cianidanol, also known as (+)-catechin; (+)-3,3′4′,5,7-flavanepentol; 3,3′,4′, 5,7-pentahydroxyflavane, [R,3S]-2-[3,4-dihydroxyphenyl]-3,4-dihydro-1[2H]- benzopyran-3,4,7-triol, or 2-(3,4-dihydroxyphenyl)-3,4-dihydro-(2r-trans)-2h- 1-benzopyran-7-triol, is a flavonoid associated with a variety of blood disorders. An association of cianidanol and immune hemolytic anemia and thrombocytopenia has been suggested in numerous case reports. In several reports, the presence of flavonoid-dependent antibodies against red blood cells is evident with cianidanol combining with erythrocyte membranes and inducing development of autoantibodies and antibodies.Several patients had platelet antibodies with increased serum-bindable IgG values without the presence of cianidanol and several with high platelet-associated IgG levels.Antibodies against cianidanol were not detectable in certain cases.The hemolytic anemia caused acute renal failure in some patients and can be life threatening, leading to fatalities. Upon cessation of treatment with cianidanol, hematology appears to return to normal over time.
These side effects do not appear to be specific to cianidanol as the formation of IgE and IgG antibodies to troxerutin (Venoruton) and silymarin (Kegalon) was also evident with patients presenting with fever, skin eruptions, and intravascular hemolysis often demonstrating high IgG titers.Furthermore, cianidanol-induced hemolysis might also occur after sensitization by other flavonoids including rutin. | | Definition | ChEBI: The (+)-enantiomer of catechin and a polyphenolic antioxidant plant metabolite. | | Brand name | Ausoliver;Cirramina;Transepar. | | World Health Organization (WHO) | Cianidanol, which is extracted from the tropical plant Uncaria
gambir, was introduced in 1976 as an adjunct in the treatment of liver disorders.
Following a cluster of cases of haemolytic anaemia reported in 1985 from Naples,
Italy, four of which were fatal, the company suspended sales worldwide. Although
subsequently reintroduced in Switzerland and France for the treatment of acute
and chronic hepatitis-B, it was later definitively withdrawn in Switzerland on
detailed reassessment and the manufacturer has now withdrawn the product
worldwide. | | General Description | (+)-Catechin is an important beneficial compound, belonging to the flavonoid class, which is present abundantly in red wine. |
| | Cianidanol Preparation Products And Raw materials |
|