Current Location: HOME >> Enterprise Certification
Contact information
| Country: | |
|---|---|
| Tel: | |
| Mobile: | 17315815539 |
| E-mail: | |
| QQ: | |
| Skype: | Chat Now! |
Gold product
- CAS:845771-78-0
- Purity:
- CAS:875356-43-7
- Purity:
- CAS:174722-31-7
- Purity:
Company Profile
Shanghai Minbiotech Co. Ltd. locates at Shanghai which is a prosperous city in China. Minbiotech has about 60 staff, and 20 of them are work for R&D, 90% of staff are with bachelor or mater education background. Minbiotech insists on pharmaceutical innovation and will always focus on its brand to meet the customer requirements by quality and cost.
Enterprise Basic Information

Enterprise Certification
 |
|
| Type of Enterprise: | Limited Liablity Company |
| Legal Representative: | Hou Zuohui |
| Registered Capital: | 1,000,000.0RMB |
| Founded Date: | 2020-05-07 |
| Registered Address: | Floor 1-2, No.7, 1088 Mingcheng Road, Fengxian District, Shanghai |
| Term of Validity: | 2020-05-07—2040-05-06 |
| Business Scope: | Technology service, technology development, technology consultant, technology tranfer, technology promotion. Chemical products selling, biological products selling, chemical products selling |
| Staff number: | 50-100 People |
| Number of R & D department: | 20-50 |
| Annual turnover: | RMB 1 million-3 million |
| Main consumer market: | Pharmaceutical factory Material factory trading company Medical materials factory |
| Main marketing location: | Turkey Russia Japan United States India |
| Main product or service: | antibodies, APIs |
Manufacturer
| Marketing : | Shanghai Minbiotech Co., Ltd. |
| Address: | Floor 1-2, No.7, Mingcheng Road 1088, Fengxian District, Shanghai |
| Area: | 500㎡ |
| Pictures of scenes: | |
|
|
|
| Marketing : | Shandong Elite Bio-Pharm Technology Co., Ltd. |
| Address: | NO.A2 Advance chemical basis, Economic development zone, Shanghe District, Jinan City, Shandong |
| Area: | 10,000㎡ |
| Equipment: | chromatography column |
| Pictures of scenes: | |
|
|
| Brief description : | Our company has a strict regulation on quality control. We have about 20 staff in quality department who will record every manufacture and test. All the records will be saved to review the products quality in case of quality problems occures. |
| Quality inspection equipment: | |
|
|
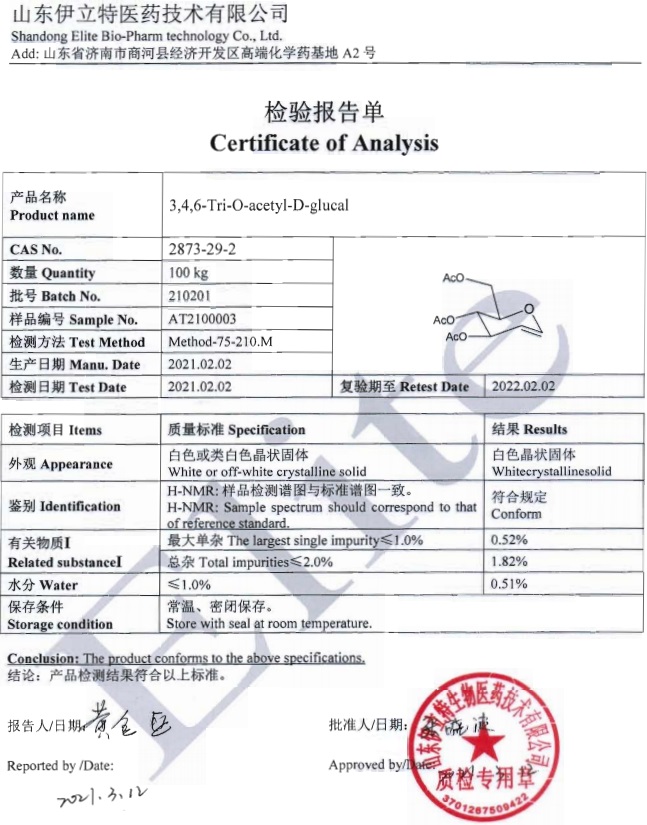
| Certificate of accreditation: | |
|
|
| Staff composition for example: | |
| Professional skills: | |
| Pictures of scenes: | |