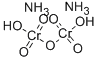
?????? ??? C??? ??, ??, ??
??? ??
Ammonium dichromate is a combustible,
orange-red crystalline solid which is used in solution.
??
Bright orange-red monoclinic crystals; odorless; hygroscopic; decomposes at 180°C; density 2.115 g/cm
3 at 25°C; readily dissolves in water (26.67 g/100 g at 20°C).
??
Ammonium dichromate is used in photo engraving, dye mordant, pigments, pickling, photography, process engraving, lithography, pyrotechnics, glazes for porcelain and china wares. It acts as a catalyst and a source of nitrogen in the laboratory and as the photoactive site. It is also used in the preparation of alizarin and chrome alum. Further, it is used in leather and oil industry for tanning and purification respectively. It is involved in the production of the phosphor raster television screens and other devices.
?? ??
Ammonium dichromate is a bright orange red crystalline solid. Ammonium dichromate is readily ignited and burns producing a voluminous green residue. If heated in a closed container, the container may rupture due to the decomposition of the material. Ammonium dichromate may also act as a strong oxidizing agent if mixed with or contaminated with combustible material. Ammonium dichromate is soluble in water.
??? ?? ??
Water soluble.
?? ???
Ammonium dichromate is an oxidizing reagent, Ammonium dichromate readily reacts with reducing materials, in large quantity Ammonium dichromate may produce a violent reaction. Direct exposure to heat or shock will explode it. When heated to decomposition Ammonium dichromate emits toxic fumes of ammonia and nitrogen oxides [Sax, 9th ed., 1996, p. 205].
???
Dusts and solutions are toxic, irritating to
eyes and skin; dangerous fire risk. Strong oxidizing
agent may explode in contact with organic materi-
als. TLV: 0.05 mg(Cr)/m
3
; Confirmed human car-
cinogen.
????
Inhalation causes irritation or ulceration of the mucous membranes of the nose, throat or respiratory tract. Respiratory irritation can produce symptoms resembling those of asthma. Continuing irritation of the nose may lead to perforation of the nasal septum. External contact can cause eye irritation and conjunctivitis, irritation and ulceration of skin wounds, and rash or external ulcers. If ingested, irritates mucous membrane and causes vomiting.
Safety Profile
Confirmed human carcinogen. Poison by inhalation, ingestion, skin contact, and subcutaneous routes. See also CHROMIUM COMPOUNDS. An unstable oxidizer. Moderately flammable; reacts with reducing agents.
??? ??
It is used in dyeing, leather tanning and
to make fireworks and chromic oxide; in lithography and photoengraving;
in manufacture of special mordants and catalysts.
?? ??
UN1439 Ammonium dichromate, Hazard Class:
5.1; Labels: 5.1-Oxidizer.
Purification Methods
It crystallises from weak aqueous HCl (ca 1mL/g). It decomposes rapidly on heating. (Possible carcinogen and is POISONOUS)
? ???
An unstable oxidizer; freezing/melting
point5(decomposes below MP) 180 C; decomposition
becomes self-sustaining and violent at about 225 C. Contact
with combustible, organic or other easily oxidized materials,
strong acids; hydrazine and other reducing agents; alcohols,
sodium nitrite may cause fire and explosions.
??? ??
Consult with environmental
regulatory agencies for guidance on acceptable disposal practices.
Generators of waste containing this contaminant
(≥100 kg/mo) must conform with EPA regulations governing
storage, transportation, treatment, and waste disposal. Add a
large volume of a reductant solution (hypo, bisulfite or ferrous
salt and acidify with sulfuric acid). Neutralize when reduction
is complete and flush to sewer with large volume of water.
?????? ??? ?? ?? ? ???
???
?? ??