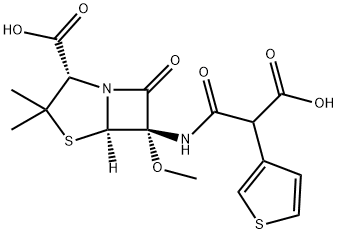
ChemicalBook >?? ???? >API>???>???? ?>(2S,5R,6S)-6-[(3-Hydroxy-3-oxo-2-thiophen-3-ylpropanoyl)amino]-6-methoxy-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
(2S,5R,6S)-6-[(3-Hydroxy-3-oxo-2-thiophen-3-ylpropanoyl)amino]-6-methoxy-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
|
|
(2S,5R,6S)-6-[(3-Hydroxy-3-oxo-2-thiophen-3-ylpropanoyl)amino]-6-methoxy-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid ??
- ?? ?
- 761.9±60.0 °C(Predicted)
- ??
- 1.60±0.1 g/cm3(Predicted)
- ?? ?? (pKa)
- 2.46±0.50(Predicted)
??
(2S,5R,6S)-6-[(3-Hydroxy-3-oxo-2-thiophen-3-ylpropanoyl)amino]-6-methoxy-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid C??? ??, ??, ??
??
Antibacterial.Antimicrobial activity
The introduction of the 6-α-methoxy group has resulted in loss of activity against Gram-positive cocci and anaerobic Gram-negative bacilli, but it is active against enterobacteria (MIC 1–8 mg/L), H. influenzae and Mor. catarrhalis, with somewhat elevated MICs against carbapenemase- producing isolates of K. pneumoniae (MIC 16–64 mg/L) and Esch. coli (modal MICs 8–16 mg/L). In most cases, β-lactamase-positive and negative strains are equally susceptible. In contrast to the structurally related ticarcillin, it is inactive against Ps. aeruginosa, but Burkholderia cepacia, Ps. acidovorans and Aeromonas spp. are susceptible (MIC 4 mg/L). Most Acinetobacter spp. are resistant, and Ser. marcescens exhibits variable susceptibility.It is bactericidal at concentrations 2–4 times the MIC; filaments formed at lower concentrations slowly lyse at higher drug levels. Temocillin consists of diastereoisomers. The naturally predominant R epimer is more rapidly bactericidal than the S epimer. It is highly resistant to most bacterial β-lactamases, including those that confer resistance to extended-spectrum cephalosporins. It is hydrolyzed by β-lactamases produced by Flavobacterium spp. and by those of Bacteroides spp.
Pharmacokinetics
Oral absorption: NegligibleCmax 1 g intramuscular injection: 70 mg/L
1 g rapid intravenous infusion: 172 mg/L after 5 min
Plasma half-life: 4.3–5.4 h
Plasma protein binding: 85%
Absorption and distribution
It is not absorbed when given orally and must be administered parenterally. Relatively high protein binding, together with its distribution in a volume less than the extracellular fluid, accounts for its relatively low renal clearance and subsequent high urinary concentrations that may be effective against some Enterobacteriaceae resistant to other β-lactam antibiotics. In artificial blister fluid and peritoneal fluid, concentrations reach 50% of the peak plasma level; in lymph, concentrations reach 25–60% of the simultaneous plasma level, with a similar half-life. The R epimer differs from the S epimer in lower protein binding, a 25% greater volume of distribution and a 60% shorter half-life.
Metabolism and excretion
Elimination is principally in the glomerular filtrate, with 80% of the dose appearing in the urine in the first 24 h. A small amount is disposed of in the bile and by degradation. Elimination declines in parallel with renal function, the half-life reaching 30 h in patients with creatinine clearance below 5%.
Clinical Use
Severe infection with susceptible bacteria, including urinary and respiratory tract infections, peritonitis and septicemia.???
As with all penicillins, hypersensitivity reactions, including serious anaphylactic responses, may occur. It is generally well tolerated and administration of 4 g intravenously every 12 h produced no significant effect on template bleeding time, prothrombin time or ADP-induced platelet aggregation.(2S,5R,6S)-6-[(3-Hydroxy-3-oxo-2-thiophen-3-ylpropanoyl)amino]-6-methoxy-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid ?? ?? ? ???
???
?? ??
(2S,5R,6S)-6-[(3-Hydroxy-3-oxo-2-thiophen-3-ylpropanoyl)amino]-6-methoxy-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid ?? ??
???( 22)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49374 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 7724 | 58 |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 |
sales@afinechem.com | China | 15354 | 58 |
| BOC Sciences | 16314854226; +16314854226 |
inquiry@bocsci.com | United States | 19741 | 58 |
| Hefei Hirisun Pharmatech Co., Ltd | +8615056975894 |
shawn@hirisunpharm.com | CHINA | 9911 | 58 |
| Amadis Chemical Company Limited | 571-89925085 |
sales@amadischem.com | China | 131957 | 58 |
| Shanghai T&W Pharmaceutical Co., Ltd. | +86 21 61551611 |
China | 9891 | 58 | |
| Henan Jiuhua Biological Technology Co., Ltd. | 15738884691 153248764872949584887 237654463 |
jiuhuabiochem@163.com | China | 2750 | 58 |
| Nanjing crow LuNing pharmaceutical technology co., LTD | 13382066392 |
CHINA | 4877 | 58 | |
| Wuhan Yingnuo Pharmaceutical Technology Co., Ltd. | +86-027-59232304 15387063101 |
2881924050@qq.com | China | 9951 | 58 |