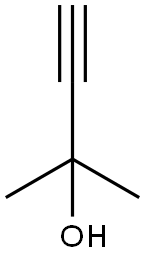
3-??-1-??-3-?
|
|
3-??-1-??-3-? ??
- ???
- 3 °C
- ?? ?
- 104 °C(lit.)
- ??
- 0.868 g/mL at 25 °C(lit.)
- ???
- 15 mm Hg ( 20 °C)
- ???
- n
20/D 1.42(lit.)
- ???
- 77 °F
- ?? ??
- Store below +30°C.
- ???
- ??? ?? ?????.
- ??? ??
- ?? ?? ??
- ?? ?? (pKa)
- 13.34±0.29(Predicted)
- ??
- ???? ???-?????? ????
- ??????(pH)
- 7 (H2O, 20℃)
- ????
- 1.8-16%(V)
- ???
- ?? ??
- Merck
- 14,6034
- BRN
- 635746
- ???
- ????. ???. ?? ???? ???? ????.
- LogP
- 0.318 at 20-25℃
- CAS ??????
- 115-19-5(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xn | ||
|---|---|---|---|
| ?? ???? ?? | 10-22-41 | ||
| ????? | 26-39-16 | ||
| ????(UN No.) | UN 1987 3/PG 2 | ||
| WGK ?? | 1 | ||
| RTECS ?? | ES0810000 | ||
| ?? ?? ?? | 662 °F | ||
| TSCA | Yes | ||
| ?? ?? | 3 | ||
| ???? | II | ||
| HS ?? | 29052990 | ||
| ?? | LD50 orally in Rabbit: 1420 mg/kg LD50 dermal Rat > 2000 mg/kg | ||
| ???? ?? | KE-23667 |
3-??-1-??-3-? C??? ??, ??, ??
??? ??
colourless to light yellow liquid.Methylbutynol (3-Methyl butynol) is a colorless, transparent, and aromatic liquid that can be consumed at room temperature. It is soluble in water and fatty acids and miscible with acetone, benzene, carbon tetrachloride, and petroleum ether but insoluble in ammonia. Methylbutynol and water form an azeotrope with an azeotropic point of 91 °C in the methylbutynol composition of 74% and the water composition of 26%. Methylbutynol is chemically active and has the common reactions of alcohol groups and unsaturated triple bonds, such as addition, polymerization, esterification, and oxidation reactions.
??
Stabilizer in chlorinated solvents, viscosity reducer and stabilizer, electroplating brightener, intermediate.?? ??
3-Methyl butynol is produced from Acetone and Acetylene in liquid Ammonia with Sodamide (or in Cellosolve with Potassium hydroxide). The process yields Methylbutynol which can be hydrogenated to Methylbutenol.?? ??
Colorless to straw yellow liquid.??? ?? ??
Highly flammable.?? ???
3-Methyl butynol is an alcohol. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides.???
Flammable, dangerous fire risk.????
Contact will cause eye and skin irritation. Vapor exposure may cause eye and respiratory tract irritation.????
Special Hazards of Combustion Products: Irritating and toxic gases, such as carbon dioxide and carbon monoxide, may be produced in fire.Synthesis
Three parts of the strong basic anion exchange resin Amberlite IRA400 were suspended in 30 parts of 7% sodium hydroxide solution to change to OH type. After washing to neutral with water, the resin was suspended in methanol, and after 15–60 min, methanol was discharged. The treatment with methanol was repeated as described above until the water content of the effluent methanol was less than 0.1% and the water content of the ion exchange resin was less than 0.2%. The tubular reactor was filled with the ion exchange resin, and then acetone was saturated with acetylene, and liquid ammonia was introduced into the reactor at 2.5 MPa and 40 °C. Excess acetylene in reactants was used to reduce the side reaction of acetone. After ethynylation, the reaction solution was subjected to a flash evaporation at 50 °C. The remaining solution was distilled to obtain methylbutynol (3-methyl butynol).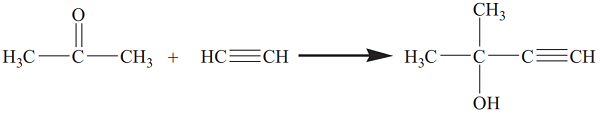
3-??-1-??-3-? ?? ?? ? ???
???
???
????
1-Butyne, 3-(1-ethoxyethoxy)-3-methyl-
2,5-???-3-??-2,5-??
2-METHYL-4-TRIMETHYLSILYL-3-BUTYN-2-OL
????
ETHYNYLMAGNESIUM BROMIDE
??????????
?????
???????
?? ??
3-??-1-??-3-? ?? ??
???( 317)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5893 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8816 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12829 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29791 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21639 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1803 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 |
sales@amoychem.com | China | 6383 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 |
sales@alchempharmtech.com | United States | 63687 | 58 |