ChemicalBook >?? ???? >????
????
|
|
???? ??
- ?? ?
- 535.9±50.0 °C(Predicted)
- ??
- 1.63±0.1 g/cm3(Predicted)
- ???
- DMSO:60.0(Max Conc. mg/mL);140.7(Max Conc. mM)
- ??? ??
- ??
- ?? ?? (pKa)
- 10.61±0.70(Predicted)
- ??
- Light yellow to brown
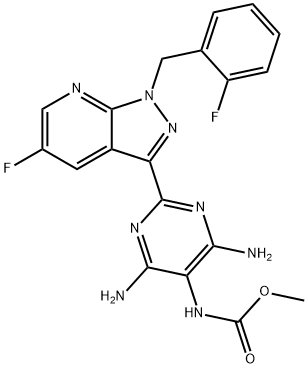
- InChI
- InChI=1S/C19H16F2N8O2/c1-31-19(30)25-14-15(22)26-17(27-16(14)23)13-11-6-10(20)7-24-18(11)29(28-13)8-9-4-2-3-5-12(9)21/h2-7H,8H2,1H3,(H,25,30)(H4,22,23,26,27)
- InChIKey
- QZFHIXARHDBPBY-UHFFFAOYSA-N
- SMILES
- C(OC)(=O)NC1=C(N)N=C(C2C3=CC(F)=CN=C3N(CC3=CC=CC=C3F)N=2)N=C1N
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
???? C??? ??, ??, ??
??
Vericiguat is a novel oral soluble guanylate cyclase (sGC) stimulator.- Soluble guanylate cyclase (sGC) is an enzyme that is activated by nitric oxide (NO). The activation initiates a signaling cascade which converts guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP). cGMP levels increase & protein kinase G (PKG) is activated, resulting in a decrease of intracellular free Ca++ -> vascular smooth muscle cell relaxation.
- However, individuals with HF have reduced NO levels. Vericiguat, as a sGC stimulator, increases the enzymatic activity of sGC to generate cGMP independently of NO and enhances sGC sensitivity to endogenous NO.
Vericiguat is the 2nd drug in this class and follows riociguat ADEMPAS, which has been approved for treating pulmonary arterial hypertension.
SOCRATES-REDUCED was a phase II dose-finding trial of vericiguat in HF-rEF. The primary endpoint, change in NTproBNP over 12 weeks, was not statistically significant compared to placebo. A secondary exploratory analysis suggested a dose-response relationship in which higher doses of vericiguat were associated with greater reductions in NTproBNP levels.
??
Vericiguat is a novel oral soluble guanylate cyclase (sGC) stimulator that enhances the cyclic guanosine monophosphate (GMP) production pathway, by directly stimulating soluble guanylate cyclase activity, as well as sensitizing soluble guanylate cyclase to endogenous NO. It was initially developed for potential to reduce mortality and morbidity associated with chronic heart failure with reduced ejection fraction.Clinical Use
Vericiguat was advanced to clinical evaluation, as an oral therapy for chronic heart failure, either with reduced ejection fraction (HFrEF), or preserved EF (HFpEF). In mid-June 2020 Merck announced that the FDA had granted priority review status to vericiguat and based on this. The FDA approved vericiguat in January 2021, to reduce the risk of cardiovascular death, heart failure re-hospitalisation, or the requirement for outpatient intravenous diuretics, in patients with symptomatic chronic heart failure and ejection fraction less than 45%. This approval was based on efficacy data arising from the Phase 3 trial NCT02861534 (a.k.a. the VICTORIA trial).???? ?? ?? ? ???
???
?? ??
???? ?? ??
???( 161)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Shijiazhuang Dingmin Pharmaceutical Sciences Co., Ltd. | +86-0311-67591193 +8613931880626 |
sales02@dingminpharma.com | China | 252 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3882 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 |
info@fortunachem.com | China | 5986 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 |
sales@sdperfect.com | China | 294 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +86-0571-28186870; +undefined8613073685410 |
sales@ichemie.com | China | 998 | 58 |
| BOC Sciences | +1-631-485-4226 |
inquiry@bocsci.com | United States | 19553 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 32002 | 58 |
| Changzhou Xuanming Pharmaceutical Technology Co., Ltd. | +8615995072465 |
sales@xuanmingchem.com | China | 914 | 58 |
| Shenzhen Shengda Pharma Limited | 755-85269922 +8613424394241 |
sales@shengdapharm.com | CHINA | 310 | 58 |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 |
sales@sarms4muscle.com | China | 10473 | 58 |