???? ?????
|
|
???? ????? ??
- ???
- 1700°C
- ??
- 1.818
- ???
- g/100g ?? H2O: 30.69(0°C), 34.51(28°C); ???, MgS2O3 · 6H2O [KRU93]; ??? ??? [MER06]
- Merck
- 14,5694
- CAS ??????
- 10124-53-5(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xi | ||
|---|---|---|---|
| ?? ???? ?? | 36/37/38 | ||
| ????? | 26-36 | ||
| WGK ?? | 3 | ||
| RTECS ?? | XN6550000 | ||
| ?? | mouse,LD50,subcutaneous,850mg/kg (850mg/kg),Arzneimittel-Forschung. Drug Research. Vol. 5, Pg. 376, 1955. | ||
| ???? ?? | KE-22758 |
| ????(GHS): |

|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ?? ?: | Warning | ||||||||||||||||||||||||||||
| ??·?? ??: |
|
||||||||||||||||||||||||||||
| ??????: |
|
???? ????? C??? ??, ??, ??
??
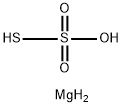
Magnesium thiosulfate has the molecular formula of MgS2O3 and the molecular weight of 136.4352 g/mol. It is also known as “hyposulfite”. It can be prepared by the reaction of sodium thiosulfate upon the chloride in water:MgCl2+Na2S2O3? MgS2O3+2NaCl
Since magnesium thiosulfate is soluble in water (50 g/100 ml at 20°C), the solution must be evaporated at a low temperature to crystallize the product (salt-out temperature ~15°F (-9.4°C). A hexahydrate, MgS2O3· 6H2O, is the result.
These are colorless crystals that lose water at 170°C to form the trihydrate. Further heating to 420°C then forms the anhydrate. Further heating to 600°C will cause the production of magnesium sulfate, sulfur and oxides of sulfur:
2MgS2O3+2O2+heat? 2MgSO4+S+SO2 Magnesium thiosulfate is considered to have a low toxicity to humans. It has been used to remove ozone from either a water stream, such as a drinking water or wastewater stream being treated with ozone, or scrubbing ozone from a gaseous stream by contacting the gaseous stream with solid magnesium thiosulfate. It has also been used to remove chlorine gas from a water stream by contacting the stream with magnesium thiosulfate solution. In yet another aspect, a method of scrubbing chlorine from a gaseous stream comprises contacting the gas stream with solid magnesium thiosulfate.
Magnesium thiosulfate is not commonly referenced in scientific literature. The few articles that prevail discuss technically specific physical properties of the end product itself. Synthetic methods are described in some Japanese patents and there is mention of MgTS in a short German description in Handbuch der Anorganischen Chemie, vol. 11, 1984. Both refer to adding sulfur to magnesium sulfite to produce magnesium thiosulfate. Neither reference describes the pH nor physical consistency of the “sulfite” intermediate, although the German article does note the “color change” observed as SO2 is purged into MgO: the slurry becomes yellow.
??? ??
colorless or white crystal(s) [MER06]???? ????? ?? ?? ? ???
???
?? ??
???? ????? ?? ??
???( 82)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5878 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5906 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29808 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 |
1057@dideu.com | China | 3889 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 |
info@dycnchem.com | China | 52849 | 58 |
| CD Chemical Group Limited | +8615986615575 |
info@codchem.com | China | 20342 | 58 |