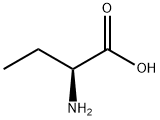
L(+)-2-Aminobutyric acid
|
|
L(+)-2-Aminobutyric acid ??
- ???
- 300 °C
- ?? ?
- 215.2±23.0 °C(Predicted)
- ??
- 21.6 º (c=2, 5N HCl)
- ??
- 1.2300 (estimate)
- ???
- 1.4650 (estimate)
- ?? ??
- room temp
- ???
- 22.7g/100mL(22°C)
- ?? ?? (pKa)
- 2.29(at 25℃)
- ??? ??
- ??? ??
- ??
- ???? ????
- ???? ??
- Porcine kidney
microbial
plant
- ???
- 22.7g/100mL(22ºC)
- Merck
- 14,428
- BRN
- 1720935
- InChIKey
- QWCKQJZIFLGMSD-VKHMYHEASA-N
- CAS ??????
- 1492-24-6(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
L(+)-2-Aminobutyric acid C??? ??, ??, ??
??? ??
White Crystalline Solid??
ChEBI: An optically active form of alpha-aminobutyric acid having L-configuration.?? ??
L-2-Aminobutyric acid is synthesized from L-threonine and L-aspartic acid through a ?-transamination reaction. It is an L-alanine analogue with an ethyl side chain.Synthesis
The preparation of l-2-aminobutyric acid (l-ABA) is mainly achieved by chemical synthesis or enzymatic conversion. In chemical methods, synthesis of l-ABA has been extensively reported, including ammonolysis of α-halogen acid, reduction reaction, ammoniation hydrolysis reaction and butanone acid reduction. However, the obvious disadvantages of chemical synthesis, such as poor selectivity, harsh reaction conditions, various byproducts, and the difficulty in separation and purification, limited its development. It is reported that l-ABA was synthesized in a transamination reaction from α-ketobutyric acid and l-aspartic acid as substrates using aromatic aminotransferase or produced from α-ketobutyric acid and benzylamine using ω-aminotransferase. l-ABA could also be produced from the reduction of α-keto acids with l-leucine dehydrogenase or glutamate dehydrogenase[1].Purification Methods
Crystallise butyrine from aqueous EtOH, and the melting point depends on heating rate but has m 303o in a sealed tube. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 2399 IR: 2401 1961, Beilstein 4 III 1294, 4 IV 2584.]L(+)-2-Aminobutyric acid ?? ?? ? ???
???
?? ??
L(+)-2-Aminobutyric acid ?? ??
???( 659)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Jiangxi Chiyan Biopharmaceutical Technology Co.,Ltd. | +86-18616643091 +86-18616643091 |
info@rochipharma.com | China | 246 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 |
Mandy@hangyubiotech.com | China | 10986 | 58 |
| Beijing Toachem Chemical Co.,Ltd | +8618311365218 |
sales@toachem.com | China | 184 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8803 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12825 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +86-0571-28186870; +undefined8613073685410 |
sales@ichemie.com | China | 1015 | 58 |
| Sichuan HongRi Pharma-Tech Co.,Ltd | +86-028-64841719 +8617390183901 |
daisy@enlaibio.com | China | 1106 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +8615350851019 |
admin@86-ss.com | China | 1001 | 58 |
| Hebei Zhuanglai Chemical Trading Co Ltd | +86-16264648883 +86-16264648883 |
niki@zlchemi.com | China | 3712 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29792 | 60 |
L(+)-2-Aminobutyric acid ?? ??:
???? ?? ???? ??? ??-???-N-??? ? N-???-DL-???? L-???? ???? 1-?????????????
DL-2-AMINOBUTYRIC ACID
Hydrocortisone-17-butyrate
r-Amino Butryric Acid
(2S)-2-(2-Oxopyrrolidin-1-yl)butanoic acid
4-CHLOROBUTYRIC ACID
ETHYL 4-CHLOROBUTYRATE
Gamma Butyrolactone
(R)-(-)-2-AMINOBUTANAMIDE HYDROCHLORIDE, 97%
Ascoric Acid