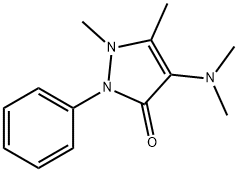
Aminopyrine C??? ??, ??, ??
??? ??
White leaf-like crystals or crystalline powder. Odorless, slightly bitter taste. It is stable in the air, but will deteriorate in sunlight. When there is moisture, it is easy to chemically react with weak oxidants. Soluble in alcohol, chloroform, benzene and ether, soluble in water. The solubility in water increases with the addition of sodium benzoate. Aqueous solutions are weakly alkaline to litmus. The melting point is 107-109°C.
??
Aminopyrine is used as an antipyretic and analgesic drug. It belongs to the pyrazolone derivatives having a most toxic and most dangerous analgesic effect and it is a non-narcotic drug. Due to strong adverse effects, its single medicine preparation is gradually replaced by compound preparation.
?? ??
Aminopyrine is synthesized from 4-Aminoantipyrine by catalytic hydrogenation (alkylation). Water is firstly added to the dissolving tank, heated to 50-60°C, put into 4-Aminoantipyrine under stirring, and poured into the hydrogenation tank after complete dissolving. Wash the dissolving tank with water, mix it with the nickel catalyst, and then add it to the hydrogenation tank. The hydrogenation tank was evacuated, then the vacuum valve was closed, hydrogen was introduced, and stirring was started. When the pressure rose to 0.245MPa, the hydrogen was stopped. Add formaldehyde and continue to feed hydrogen. The speed of adding formaldehyde is based on the standard of 1.8-2 cubic meters of hydrogen absorption per 5L of formaldehyde, and the reaction temperature is controlled at 60-85°C. After passing the test, filter by pressure, cool the filtrate to below 25°C, and separate out crystals, which are then filtered to obtain the crude aminopyrine. The crude aminopyrine, ethanol, and activated carbon were heated to 75-80°C, stirred for 1 hour, and filtered under pressure. The filtrate was cooled to 10°C, crystals were precipitated, filtered, washed with ethanol, and air-dried to obtain aminopyrine.
??
ChEBI: Aminopyrine is a pyrazolone that is 1,2-dihydro-3H-pyrazol-3-one substituted by a dimethylamino group at position 4, methyl groups at positions 1 and 5 and a phenyl group at position 2. It exhibits analgesic, anti-inflammatory, and antipyretic properties. It has a role as a non-steroidal anti-inflammatory drug, a non-narcotic analgesic, an antipyretic, an environmental contaminant and a xenobiotic. It is a tertiary amino compound and a pyrazolone.
World Health Organization (WHO)
Aminophenazone, a pyrazolone derivative, has been used as an
antiinflammatory and analgesic agent for over a century. Its use has been
associated with cases of bone marrow depression and agranulocytosis and more
recently it has been claimed to have a carcinogenic potential. Products containing
aminophenazone have been formally withdrawn in many countries and marketing
has been voluntarily suspended in others. Elsewhere, however, proprietary
preparations containing this ingredient may remain available.
(Reference: (WHODI) WHO Drug Information, 3, 9, 1977)
?? ??
Small colorless crystals or white crystalline powder. Aqueous solution slightly alkaline to litmus. pH (5% water solution) 7.5-9. Odorless. Slightly bitter taste.
??? ?? ??
Water soluble.
?? ???
Aminophenazone is sensitive to exposure to light. Aminophenazone is readily attacked by mild oxidizing agents in the presence of water. Aminophenazone is incompatible with acacia, apomorphine, aspirin, chloral hydrate, iodine and tannic acid.
????
Flash point data for Aminophenazone are not available; however, Aminophenazone is probably combustible.
Safety Profile
Human poison by
unspecified route. Experimental poison by
ingestion, subcutaneous, intramuscular,intravenous, and intraperitoneal routes.
Moderately toxic by parenteral route.
Experimental teratogenic and reproductive
effects. Questionable carcinogen when
mixed with NaNO2 (1:l). Mutation data
reported. Can cause bone marrow
depression resulting in leucopenia. Has been
implicated in development of aplastic
anemia. A tranquilizer. When heated to
decomposition it emits toxic fumes of NOx.
Purification Methods
It crystallises from pet ether, sublimes between 80o and 90o, and forms metal complexes. [Beilstein 25 H 452, 25 III/IV 3555.]
Aminopyrine ?? ?? ? ???
???
?? ??