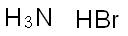??????
|
|
?????? ??
- ???
- 452 °C (lit.)
- ?? ?
- 396 °C/1 atm (lit.)
- ??
- 2.43 g/mL at 25 °C (lit.)
- ???
- 1 mm Hg ( 198.3 °C)
- ?? ??
- Inert atmosphere,Room Temperature
- ???
- H2O: 1 M at 20 °C, ??, ??
- ?? ?? (pKa)
- -1.03±0.70(Predicted)
- ??? ??
- ??/??
- ??
- ???
- Specific Gravity
- 2.429
- ??
- ?? ??
- pH ??
- 4.5 - 6.0
- ??????(pH)
- 5.0-6.0 (25℃, 50mg/mL in H2O)
- ???
- 97g/100mL(25℃)
- ??
- Hygroscopic
- Merck
- 14,505
- Dielectric constant
- 7.2(0.0℃)
- ???
- ????. ?, ??? ??, ??? ??, ???? ???? ??? ???? ????.
- LogP
- -1 at 25℃
- CAS ??????
- 12124-97-9(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xn,Xi | ||
|---|---|---|---|
| ?? ???? ?? | 22-36/37/38-36 | ||
| ????? | 22-24/25 | ||
| WGK ?? | 2 | ||
| RTECS ?? | BO9155000 | ||
| TSCA | Yes | ||
| HS ?? | 28275900 | ||
| ?? ?? ??? | 12124-97-9(Hazardous Substances Data) | ||
| ?? | LD50 orally in Rabbit: 2714 mg/kg | ||
| ???? ?? | KE-01639 |
?????? C??? ??, ??, ??
?? ??
??? ????? (NH4Br)? ?? ?? ?? ? ?? ??? ??? ???? ?????.?? ???? ?? ? ?? ??? biocide? ?????.
?? ??
????????
??? ???
??
Ammonium bromide is soluble in water and alcohol and is not particularly hazardous under normal conditions.??? ??
Ammonium bromide is a weak acid with a pKa of approximately 5 in water. It is an acid salt because the ammonium ion hydrolyzes slightly in water.NH4Br(s) → NH+4(aq) + Br-(aq)
It decomposes to ammonia and hydrogen bromide when heated at elevated temperatures.
??? ??
Colorless rhombic crystal; odor of ammonia; sublimes at 60°C; very soluble in cold water; decomposes in hot water; slightly soluble in alcohol; insoluble in acetone.??
Ammonium bromide is a colorless crystals used for its bromide ion, it was also used as a halide in collodion formulas and as a restrainer in alkaline developers.?? ??
Ammonium bromide can be prepared by the direct action of hydrogen bromide on ammonia. NH3 + HBr → NH4Br??
ChEBI: An ammonium salt composed of ammonium and bromide ions in a 1:1 ratio.?? ??
White odorless crystals or granules that become yellow upon exposure to air. Sinks and mixes in water.??? ?? ??
Water soluble?? ???
Bromine trifluoride reacts explosively with the following Ammonium bromide, ammonium chloride, ammonium iodide [Mellor 2 Supp. 1:165 1956].????
INHALATION: Dust irritating - disturbed behavior, sedation. EYES: Slight irritation. SKIN: Slight irritation only with repeated or prolonged contact. INGESTION: Weakness, nervousness, anorexia, confusion, hallucinations, drowsiness, irritability, ataxia, vertigo, skin rash.????
Special Hazards of Combustion Products: Material decomposes into N 2 and HBr or Br 2 under extreme temperatures.Purification Methods
It crystallises from 95% EtOH and is slightly hygroscopic.?????? ?? ?? ? ???
???
?? ??
?????? ?? ??
???( 512)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8810 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12835 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5889 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 3002 | 58 |
| Hebei Andu Technology Com.,Ltd | +86-86-17798073498 +8617798073498 |
admin@hbandu.com | China | 299 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21634 | 55 |
| Yancheng Green Chemicals Co.,Ltd | +undefined-86-25-86655873 |
info@royal-chem.com | China | 114 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29880 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 |
sales@amoychem.com | China | 6383 | 58 |