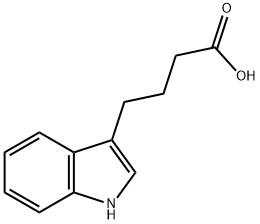
Indole-3-butyric acid
- CAS No.
- 133-32-4
- Chemical Name:
- Indole-3-butyric acid
- Synonyms
- IBA;INDOLE-3-BUTYRIC ACID;3-INDOLEBUTYRIC ACID;4-(1H-INDOL-3-YL)BUTANOIC ACID;Seradix;hormexrootingpowder;3-Indolybutyric acid;4-(3-INDOLYL)BUTYRIC ACID;Indole-3-butyric acid IBA;gamma-(3-indolyl)butyricacid
- CBNumber:
- CB3286999
- Molecular Formula:
- C12H13NO2
- Molecular Weight:
- 203.24
- MDL Number:
- MFCD00005664
- MOL File:
- 133-32-4.mol
- MSDS File:
- SDS
| Melting point | 124-125.5 °C(lit.) |
|---|---|
| Boiling point | 341.55°C (rough estimate) |
| Density | 1.1255 (rough estimate) |
| refractive index | 1.5440 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMF: 25 mg/mL; DMSO: 25 mg/mL; Ethanol: 25 mg/mL; PBS (pH 7.2): 0.1 mg/mL |
| pka | 4.83±0.10(Predicted) |
| form | Liquid |
| color | Clear colorless to pale yellow |
| Water Solubility | Soluble in water(0.25g/L). |
| Sensitive | Air Sensitive |
| Merck | 14,4965 |
| BRN | 171120 |
| Stability | Stable. Incompatible with strong oxidizing agents. Light sensitive. |
| InChIKey | JTEDVYBZBROSJT-UHFFFAOYSA-N |
| LogP | 2.300 |
| CAS DataBase Reference | 133-32-4(CAS DataBase Reference) |
| FDA UNII | 061SKE27JP |
| EPA Substance Registry System | Indole-3-butyric acid (133-32-4) |
| Pesticides Freedom of Information Act (FOIA) | Indole-3-butyric acid |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301 | |||||||||
| Precautionary statements | P264-P270-P301+P310-P405-P501 | |||||||||
| Hazard Codes | T,Xi | |||||||||
| Risk Statements | 25-36/37/38 | |||||||||
| Safety Statements | 26-36-45-38-36/37/39-28A | |||||||||
| RIDADR | UN 2811 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | NL5250000 | |||||||||
| F | 8-10-23 | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | T | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29339990 | |||||||||
| Toxicity | LD50 i.p. in mice: 100 mg/kg (Anderson) | |||||||||
| NFPA 704 |
|
Indole-3-butyric acid price More Price(44)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 57310 | Indole-3-butyric acid ≥99.0% (T) | 133-32-4 | 5G | $65.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 57310 | Indole-3-butyric acid ≥99.0% (T) | 133-32-4 | 25G | $170 | 2024-03-01 | Buy |
| Sigma-Aldrich | 57310 | Indole-3-butyric acid ≥99.0% (T) | 133-32-4 | 100G | $483 | 2024-03-01 | Buy |
| Sigma-Aldrich | 45532 | Indole-3-butyric acid PESTANAL , analytical standard | 133-32-4 | 250mg | $45.4 | 2022-05-15 | Buy |
| TCI Chemical | I0026 | 3-Indolebutyric Acid >98.0%(HPLC)(T) | 133-32-4 | 5g | $33 | 2024-03-01 | Buy |
Indole-3-butyric acid Chemical Properties,Uses,Production
Plant Growth Regulator
Indole-3-butyric acid (IBA) is a plant hormone belonging to the auxin family and assists in initiating root formation; the in vitro process is called micropropagation. Aside from accelerating root formation, it is used on various crops to stimulate flower development and the growth of fruits. This ultimately increases crop yields.Because it is similar in structure to naturally occurring substances and is used in tiny amounts, this plant growth regulator poses no known risks to humans or the environment.
Indole-3-butyric acid is not easy to be oxidized in plants and has a poor conductivity. It has similar physiological effect as indole acetic acid. It can take effect in cell division and cell growth of plants but with a less significant effect compared with indole acetic acid is mainly used to promote rooting cuttings and effectively promote cell division cambium. It has a relative long maintaining time for its efficacy and can promote more but slender adventitious roots with better efficacy when being administered together with naphthalene acetic acid.
Indole-3-butyric acid can be applied to the cuttings and root cutting of chrysanthemum and other ornamental plants for facilitating the rooting at a concentration of 0.5~1.0mg/L. However, we should not be applied to the foliage of the plants. Degradation and Metabolism: it can subject to rapid degradation in the soil.
Physical and Chemical Properties
3-Indolebutyric acid: (molecule formula: C11H12O2N) has a molecular weight of 190.22. The pure product is white crystalline solid with the melting point being 124~125 ℃. Its industrial product is white to pale yellow crystals with a melting point being 121~124 ℃. It has special smell and is toxic! It is irritant with a vapor pressure being <10μPa at 60 ℃. It is difficult to be dissolved in water with the solubility in water at 20 ℃ being 0.25g/L. It is easily soluble in benzene and soluble in other organic solvents. Its solubility (g/100ml) is: benzene> 100; acetone, ethanol, ethyl ether: 3 to 10; chloroform: 1 to 10. 3-indolebutyric acid has a low toxicity to humans and animals and is stable to the acid while forming salt in the solution of the hydroxide and carbonate compounds of the alkali metal. It can be produced from indole via Grignard reaction or by adding nitrile for hydrolysis. In Agriculture, 3-indolebutyric acid can be used as plant growth-promoting agent that can promote root growth and fruit ripening with an efficacy being stronger than indole acetic acid. Indole butyric acid is an excellent growth regulator for promoting the cutting and rooting of the cherry rootstock. You can apply rapid high-concentration dip method or soaking for treatment of cutting slips. Rapid high-concentrations dip method is through dipping the base of the cutting slips in IBA solution of a concentration of 5 × 10-4~1 × 10-2 for 5 seconds and immediately inserting the cutting slip in the bed. This method has the advantage of short processing time, even medication and excellent efficacy. After the treatment, adding simazine to the insertion beds (20.375 grams per meter) can lead to a higher rate of rooting.
Preparation
Laboratory put the mixture of indole, potassium hydroxide, and poly oxalic acid, dried tetrahydronaphthalene and γ-butyrolactone for heating under reflux to obtain the indole butyric acid. The reaction equation is as follows:

Indole, potassium hydroxide, poly oxalic acid and dried tetrahydronaphthalene were added to the reaction vessel. The mixture was stirred for half an hour at room temperature; add γ-butyrolactone for heating reflux for 4 hours. The water remaining in the reaction system is removed by the water segregator. After cooling, the solid was totally dissolved by water with the separated organic layer being acidified with hydrochloric acid for precipitating a large number of off-white solid. Use suction filtration, wash with water, and dry to give the crude indole butyric acid with the yield of the crude product being 92.6%. After recrystallisation but aqueous ethanol, you can obtain white flaky crystals that is indole-3-butyric acid with the mp being 121.5~123 ℃.

Reference: Daquan Wang (editor), “fine chemical production route” 2nd version, Beijing: Chemical Industry Press .1999 p. 260-261.
Analytical method
1. Product analysis: in the absolute ethanol, measure it at wavelength of 281nm through UV spectrophotometry (ACF Chemiefarma method).
2. Residue Analysis: use chloroform for extraction from the acid medium, and put its methanol solution together with petroleum ether (40~60 ℃) for shaking absorption, and further use high performance liquid chromatography for applying ultraviolet detection (ACF Chemiefarma France). Alternatively, you can also refer to the indole acetic acid residue analysis. First derive methyl or the trimethyl ester of silane, and then use gas chromatography for analysis.
Toxicity
It has low toxicity to humans and animals. Acute-oral rat: LD50: 5000mg/kg, mice 1760mg/kg. Carp LC50: 180mg/L (48h). It has low toxicity to bees.
Chemical Properties
The pure product is white crystals. m.p.124~125 ℃, vapor pressure <10 × 10-6Pa) (60 ℃). It is easily soluble in organic solvents but insoluble in water. It is stable to acid. The industrial product is white to pale yellow crystals with a melting point of 121~124 ℃.
Uses
Indole butyric acid (IBA) is a broad spectrum indole-class plant growth regulators and is a good rooting agent. It can promote the cuttings and rooting of herbaceous and woody ornamental plant. It can also be applied to the fruit setting of fruit as well as improving the fruit setting rate.
Chemical Properties
Indole-3-butyric acid (IBA) is a white to light yellow powder or crystalline solid with a slight characteristic odor. It is practically insoluble in chloroform, but is soluble in alcohol, ether and acetone. The solubility of IBA in water is 250 mg L-1 (EPA, 2010).
Uses
Indole-3-butyric acid is auxin-family plant hormone (phytohormone). IBA is thought to be a precursor of indole-3-acetic acid (IAA) the most abundant and the basic auxin natively occurring and functioning in plants. IAA generates the majority of auxin effects in intact plants, and is the most potent native auxin.
Uses
Used to stimulate root formation of plant clippings. Suitable for plant cell culture tested.
Definition
ChEBI: Indole-3-butyric acid is a indol-3-yl carboxylic acid that is butanoic acid carrying a 1H-indol-3-yl substituent at position 1. It has a role as a plant hormone, a plant metabolite and an auxin. It derives from a butyric acid. It is a conjugate acid of an indole-3-butyrate.
General Description
Indole-3-butyric acid is a plant hormone in the auxin family and is widely used in agriculture since it induces rooting in various plants species such as mung bean (Vigna radiata?L.) cuttings.
Biochem/physiol Actions
Indole-3-butyric acid (IBA) is a naturally occurring phytohormone auxin (plant growth regulator). It promotes root formation in cuttings but does not affect ethylene levels. IBA might be a precursor for indole-3-acetic acid (IAA) through β oxidation pathway. IBA is present in plant species like Zea mays, Pisum sativum and Arabidopsis. IBA is more potent than IAA for inducing root formation.
Purification Methods
Recrystallise the acid from H2O. It is soluble in EtOH, Et2O and Me2CO but insoluble in CHCl3. [Bowman & Islip Chem Ind London 154 1971, Jackson & Manske J Am Chem Soc 52 5029 1930, Albaum & Kaiser Am J Bot 24 420 1937.] It has also been recrystallised from EtOH/water [James & Ware J Phys Chem 89 5450 1985]. Its UV has 278 and 320nm in isoPrOH [Elvidge Quart J Pharm Pharmacol 13 219 1940].max The methyl ester has m 73-74o (from *C6H6/pet ether) and b 230o/6mm [Bullock & Hand J Am Chem Soc 78 5854 1951]. [Beilstein 22 III/IV 1128, 22/3 V 140.]
Indole-3-butyric acid Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 | sales@zhuoerchem.com | China | 2904 | 58 |
| Guangzhou TongYi biochemistry technology Co.,LTD | +8613073028829 | mack@tongyon.com | China | 2994 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 | info@fortunachem.com | China | 5975 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5889 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 997 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 973 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20285 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Hebei Andu Technology Com.,Ltd | +86-86-17798073498 +8617798073498 | admin@hbandu.com | China | 299 | 58 |
Related articles
- The Versatile Role of a Plant Growth Regulator: An Overview of Indole-3-butyric Acid's Applications and Significance
- This paper reviewed the application of indole-3-butyric acid, and its mechanism of action, practical application and recent re....
- Sep 20,2024
View Lastest Price from Indole-3-butyric acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-17 | Indole-3-butyric acid
133-32-4
|
US $10.00 / kg | 1kg | 99% | 5000 | Hebei Zhuanglai Chemical Trading Co.,Ltd | |
 |
2024-12-17 | Indole-3-butyric acid
133-32-4
|
US $999.00-666.00 / kg | 1kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-12-17 | Indole-3-butyric acid
133-32-4
|
US $999.00-666.00 / kg | 1kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD |
-

- Indole-3-butyric acid
133-32-4
- US $10.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd
-

- Indole-3-butyric acid
133-32-4
- US $999.00-666.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Indole-3-butyric acid
133-32-4
- US $999.00-666.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD