4-Acetoxystyrene Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R22:Gesundheitssch?dlich beim Verschlucken.
R38:Reizt die Haut.
R43:Sensibilisierung durch Hautkontakt m?glich.
S-S?tze Betriebsanweisung:
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
Beschreibung
4-Acetoxystyrene can be used to synthesize Poly(p-hydroxystyrene) as the main component of photoresist. The chemically amplified photoresist of the poly(p-hydroxystyrene) series is currently the mainstream photoresist product in the world, and it is one of the key technologies for processing photo-etched integrated circuits and manufacturing chips.
Chemische Eigenschaften
4-Acetoxystyrene is a clear and colorless liquid that is essentially the acetic acid ester of p-vinylphenol. It will homopolymerize readily in the same manner that styrene homopolymerizes and can also be copolymerized with styrene and with monomers which are copolymerizable with styrene. 4-Acetoxystyrene is homopolymerized and copolymerized in aqueous emulsion and, without isolation, the polymer is hydrolyzed to homopolymers and copolymers of p-vinylphenol with a base. Homopolymers and copolymers of p-vinylphenol are used as epoxy resin curing agents and in the preparation of epoxy resins by reaction with epichlorohydrin.
Verwenden
4-Acetoxystyrene is a stable Styrene monomer which can be readily polymerized and copolymerized to low, medium and high molecular weight polymers. Undergoes free radical polymerization, similar to styrene.
Application
4-Acetoxystyrene is a polymer that can be used in microlithography and is the precursor of easily derivatized p-hydroxystyrene.
synthetische
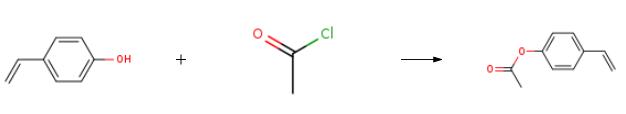
The preparation of 4-Acetoxystyrene is as follows:Add p-hydroxystyrene (120g) to a 2L four-neck bottle. triethylamine(106g), phenothiazine (1.2g), Methyl tert-butyl ether (480 g), Dry ice-ethanol bath to -5 to 0 °C, acetyl chloride (86g) was added dropwise with stirring. The internal temperature is controlled at -5 to 0 °C. After the completion of the dropwise addition, the temperature was raised at 10 to 20 °C to continue the reaction for 1 hour. Sampling analysis (central control 1, raw material After completion of the reaction, the mixture was filtered, and the cake was washed with methyl t-butyl ether (50 g × 3). The filtrate was quenched by the addition of 4 g of methanol, and the reaction was stirred for 10 minutes. After completion, phenothiazine (1.2 g) was added and the reaction mixture was concentrated.Methyl tert-butyl ether (580 g) was recovered to give a crude product (160 g).The crude product was distilled under reduced pressure to give the product, p-acetoxy styrene (142 g), yield 87.7%, and sampled for analysis (main content >99%).
Sicherheitsprofil
Moderately toxic by ingestion.Slightly toxic by skin contact. An eye irritant. A combustibleliquid. When heated to decomposition it emits acrid smokeand irritating vapors.
Advantages
Grafting 4-Acetoxystyrene (AOS) onto Polypropylene (PP) could significantly improved PP's space charge distribution, DC resistivity, and DC breakdown strength. These improvements were related to carbonyl (polar group) and benzene ring derived from AOS. As the carbonyl in AOS is introduced onto the PP chains by the grafting reaction, the homo-charge injected from the electrodes during the voltage application is trapped by the carbonyl and neutralizes the hetero-charge, decreasing hetero-charge. With the increase of grafting degree, the concentration of carbonyl increases, the corresponding charge trapping ability rises, and thus the extent of neutralizing the hetero-charge increases. The space charge is effectively suppressed. Grafting AOS onto PP significantly increases breakdown performance since AOS possesses chemical construction similar to the benzil-type voltage stabilizer. Because of delocalized π-electrons, AOS possesses a low ionizing potential and high electron affinity, making it capable of capturing the energetic electrons by collision and dissipating the energy, and the breakdown strength is improved. On the other hand, the energy of hot electrons is consumed through Fries rearrangement of AOS, weakening the attack of hot electrons on molecular chains and resulting in enhanced breakdown strength[1].
4-Acetoxystyrene Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

