Phosphorodiamidsure Chemische Eigenschaften,Einsatz,Produktion Methoden
Synthese
In the Stokes method, the synthesis of diamidophosphoric acid starts from the phenyl ester of dichlorophosphoric acid obtained from POC13 and phenol. This is converted with aqueous NH3 to the phenyl ester of diamidophosphoric acid. This ester is then saponified with Ba(OH)s ; the Ba2+ is precipitated as BaCO3, and the silver salt of diamidophosphoric acid is then obtained by precipitation with AgNO3. After reprecipitation of the silver salt, treatment with HBr results in very pure, free diamidophosphoric acid. The phenyl ester of diamidophosphoric acid can also be saponified with potassium hydroxide, and after acidification with acetic acid, the free diamidophosphoric acid can be precipitated with ethanol, yielding a somewhat less pure product.
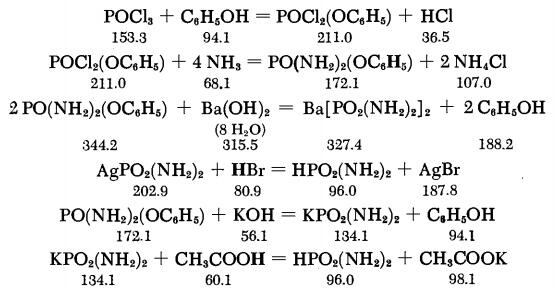
Phosphorodiamidsure Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

