Zinc Borohydride Chemische Eigenschaften,Einsatz,Produktion Methoden
Chemische Eigenschaften
Zinc borohydride ether solution is sensitive to moisture and should be stored and used under the protection of inert gas.
Verwenden
zinc borohydride is unique because of the better coordination ability of Zn2+, which imparts selectivity in hydridetransferring reactions. Zinc borohydride is moderately stable in ethereal solution and has many applications in organic synthesis. Zinc borohydride, as a nonconventional hydride transferring agent, has been reported as an efficient chemo-, regio- and stereoselective reducing agent in several complex substrates. It can be used in a range of aprotic solvents such as, THF, Et2O and DME. Several combination reducing systems of Zn(BH4)2 such as Zn(BH4)2/TMEDA, Zn(BH4)2/Me3SiCl, Zn(BH4)2/ TFA/DME, Zn(BH4 ) 2 /H2O, 1Zn(BH4 ) 2 /Al 2O3 , and Zn(BH4)2/C are interesting and have been used for different reduction purposes.
synthetische
Zinc borohydride (Zn(BH4)2) needs to be prepared by in situ reaction, anhydrous zinc chloride is reacted with sodium borohydride in anhydrous ether solvent.
Reaktionen
Zinc borohydride is often used as a reducing agent in organic synthesis, and its reducing ability is comparable to that of lithium aluminum hydride, and its selectivity is good. It can selectively reduce aldehydes, ketones, carboxylic acids and their derivatives, imines, nitriles, epoxy compounds, alkenes (alkynes), etc., and has good stereoselectivity for chiral molecules. Because the ether solution of zinc borohydride is neutral, it is very suitable for substrates containing base-sensitive functional groups such as cyano, ester, γ-lactone, etc. In addition, when saturated ketones and α,β-unsaturated ketones coexist, the reduction of saturated ketones can be preferentially achieved selectively.
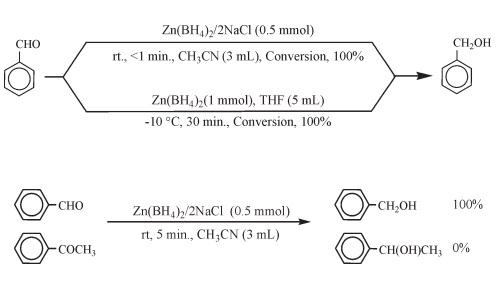
Reduction Reaction Zinc Borohydride
Zinc Borohydride Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

