Praziquantel Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R11:Leichtentzündlich.
R34:Verursacht Ver?tzungen.
S-S?tze Betriebsanweisung:
S16:Von Zündquellen fernhalten - Nicht rauchen.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
Beschreibung
Praziquantel (PZQ) is an isoquinoline derivative with most of the biological activity found in the
levo enantiomer. The compound has no activity against nematodes, but it is highly effective
against cestodes and trematodes.
Chemische Eigenschaften
White Solid
Verwenden
Praziquantel is a potent anthelmintic used against schistosome and many cestode infestations. It is used to study voltage-gated Ca2+ channels and is a potential small molecule neurogenic.
Indications
The neuromuscular effects of praziquantel (Biltricide)
appear to increase parasite motility leading to spastic
paralysis. The drug increases calcium permeability
through parasite-specific ion channels, so that the
tegmental and muscle cells of the parasite accumulate
calcium.This action is followed by vacuolization and the
exposure of hitherto masked tegmental antigens, lipidanchored
protein, and actin. Insertion of the drug into
the fluke’s lipid bilayer causes conformational changes,
rendering the fluke susceptible to antibody- and
complement-mediated assault.
Acquired resistance
There is evidence that resistance to praziquantel is emerging
in schistosomes, although there is debate as to whether treatment
failures are due to resistance or innate tolerance.
Pharmazeutische Anwendungen
A synthetic pyrazinoquinoline formulated for oral administration.
It is stable in the dry state, but hygroscopic.
Mechanism of action
Praziquantel is readily absorbed (80% in 24 hours)
after oral administration, with serum concentrations being
maximal in 1 to 3 hours; the drug has a half-life of
0.8 to 1.5 hours. Its bioavailability is reduced by phenytoin
or carbamazepine and increased by cimetidine.
Dexamethasone decreases plasma praziquantel levels
by 50%. Praziquantel is excreted by the kidneys.
Pharmakokinetik
Oral absorption: >80%
C
max 50 mg/kg oral: 1 mg/L after 1–2 h
Plasma half-life: parent drug: 1–1.5 h
metabolites: 4–6h
Plasma protein binding: 80%
Praziquantel is rapidly absorbed when given orally, but it
undergoes extensive first-pass biotransformation and the concentration
of unchanged drug in plasma is low. The major
metabolite, a 4-hydroxy derivative, retains little to no antiparasitic
activity. About 80% of the oral dose, as parent drug
and its metabolites, is excreted in the urine by the fourth day
post-treatment, 90% of this in 24 h. A higher peak plasma
concentration is achieved in infected people, but other pharmacokinetic
values are unchanged.
Clinical Use
2-(Cyclohexylcarbonyl)-1,2,3,6,7, 11b-hexahydro-4Hpyrazino[2,1-a]isoquinolin-4-one (Biltricide) is a broadspectrumagent that is effective against various trematodes (flukes). It has become the agent of choice for the treatmentof infections caused by schistosomes (blood flukes).
The drug also provides effective treatment for fasciolopsiasis(intestinal fluke), clonorchiasis (Chinese liver fluke),fascioliasis (sheep liver fluke), opisthorchosis (liver fluke),and paragonimiasis (lung fluke). Praziquantel increases cellmembrane permeability of susceptible worms, resulting inthe loss of extracellular calcium. Massive contractions andultimate paralysis of the fluke musculature occurs, followedby phagocytosis of the parasite.
Following oral administration, about 80% of the doseis absorbed. Maximal plasma concentrations are achievedin 1 to 3 hours. The drug is rapidly metabolized in theliver in the first-pass. It is likely that some of the metabolitesare also active. Praziquantel occurs as a white crystallinesolid that is insoluble in water. It is available as600-mg film-coated tablets. The drug is generally welltolerated.
Nebenwirkungen
Very few side effects have been reported. In the treatment
of cerebral cysticercosis the death of cysts in the brain may
cause local inflammation and edema, but this usually subsides
quickly. Ocular cysticercosis should not be treated
with this drug, because parasite destruction in the eye can
lead to irreparable lesions. Adverse events seen in the treatment
of schistosomiasis, including abdominal pain, nausea,
anorexia, diarrhea and mild neurological effects, are almost
certainly due to the death and disintegration of the large
adult worms.
Sicherheitsprofil
Poison by intraperitoneal route.Moderately toxic by ingestion and other routes. Humanmutation data reported. When heated to decomposition itemits toxic fumes of NOx.
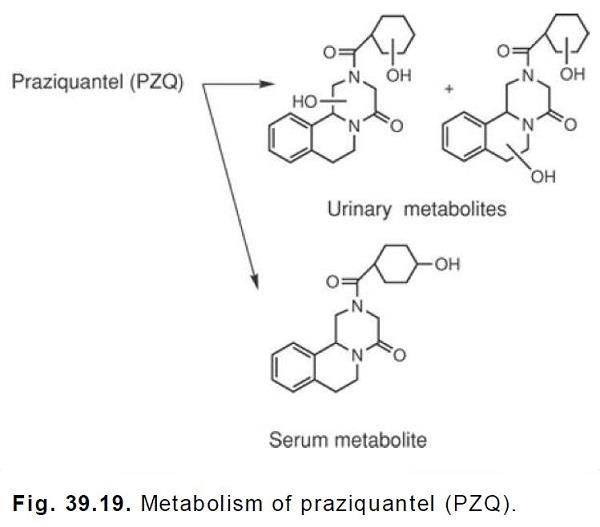
Stoffwechsel
Praziquantel is rapidly absorbed and undergoes hepatic first-pass metabolism. The metabolites are
either less active or inactive and consist of hydroxylated compounds. In the serum, the major
metabolite appears to be the monohydroxylated 4-hydroxycyclohexylcarboxylate, whereas in the
urine, 50 to 60% of the initial PZQ exists as dihydroxylated products.These
hydroxylation reactions are catalyzed by CYP2B6 and CYP3A4. The metabolites would be
expected to exist in the conjugated form in the urine.
Praziquantel Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

