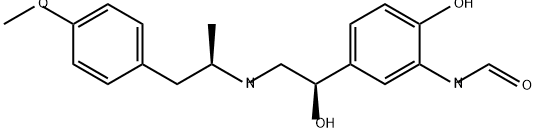
(R,R)-Formoterol
|
|
(R,R)-Formoterol Eigenschaften
- Schmelzpunkt:
- 73-75 °C
- Siedepunkt:
- 603.2±55.0 °C(Predicted)
- Dichte
- 1.233±0.06 g/cm3(Predicted)
- L?slichkeit
- 25℃: DMSO
- Aggregatzustand
- Powder
- pka
- 8.95±0.50(Predicted)
- Farbe
- White to off-white
Sicherheit
(R,R)-Formoterol Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Arformoterol, a selective long-acting b2-agonist, was launched as an inhalation solution for treatment of bronchoconstriction associated with COPD. It is the active (R,R)-enantiomer of the previously marketed b2-agonist formoterol, which is registered as a racemic mixture. Similar to other b2-agonists, the mechanism of action of formoterol and arformoterol involves activation of adenyl cyclase, leading to increased production of cyclic adenosine monophosphate (cAMP) via ATP. Increased levels of intracellular cAMP result in relaxation of the bronchial smooth muscle, and also prevent mast cells from releasing inflammatory mediators. Arformoterol has high binding affinity for the human b2 receptor (Kd 2.9 nM) and approximately 39-fold selectivity over the b1 receptor (Kd 113 nM). It is W1,000 times more potent b2 ligand than the corresponding (S,S)-enantiomer (Kd 3100 nM), and twice as potent as racemic formoterol (Kd 5.2 nM). Additionally, arformoterol acts as a full or nearly full agonist of the b2 receptor, whereas the (S,S)-enantiomer acts as an inverse agonist. Therefore, the (R,R)-enantiomer may account exclusively for the activation of b2 receptors by the racemic mixture.Verwenden
(R,R)-Formoterol (Arformoterol) (CAS# 67346-49-0) is aused in the methods for treating chronic obstructive pulmonary disease by administration of a bronchodilator using a nebulizer.Definition
ChEBI: An N-[2-hydroxy-5-(1-hydroxy-2-{[1-(4-methoxyphenyl)propan-2-yl]amino}ethyl)phenyl]formamide in which both of the stereocentres have R configuration. The active enantiomer of formoterol, it is administered by inhalation generally as the tartrate salt) as a direct-acting sympathomimetic and bronchodilator for the treatment of chronic obstructive pulmonary disease (any progressive respiratory disease that makes it harder to breathe over time, such as chronic bronchitis and emphysema).(R,R)-Formoterol Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
(R,R)-Formoterol Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 67)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 |
Mandy@hangyubiotech.com | China | 10986 | 58 |
| Standardpharm Co. Ltd. | 86-714-3992388 |
overseasales1@yongstandards.com | United States | 14332 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 |
sales@invivochem.cn | United States | 6391 | 58 |
| Shandong Risen-Sun Pharmaceutical Co., Ltd | +86-15552509998 +86-15621883869 |
liutf@jewim.com.cn | China | 251 | 58 |
| China National Standard Pharmaceutical Corporation Limited | +8615391658522 |
overseasales@yongstandards.com | China | 11922 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 43340 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +86-18621343501; +undefined18621343501 |
product@acmec-e.com | China | 33338 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 |
sales@aladdinsci.com | United States | 57505 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 |
cooperation@kean-chem.com | China | 40066 | 58 |
| T&W GROUP | 021-61551611 13296011611 |
contact@trustwe.com | China | 9895 | 58 |
- N-[2-hydroxy-5-[(1R)-1-hydroxy-2-[[(2R)-1-(4-methoxyphenyl)propan-2-yl]amino]ethyl]phenyl]formamide
- 4-[(1R)-2-[[(1R)-2-(4-Methoxyphenyl)-1-methylethyl]amino]-1-hydroxyethyl]-2-(formylamino)phenol
- N-[2-Hydroxy-5-[(1R)-1-hydroxy-2-[[(αR)-α-methyl-4-methoxyphenethyl]amino]ethyl]phenyl]formamide
- N-[2-Hydroxy-5-[(R)-1-hydroxy-2-[[(R)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]formamide
- Formamide, N-[2-hydroxy-5-[(1R)-1-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]-
- Formamide, N-[2-hydroxy-5-[1-hydroxy-2-[[2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]-, [R-(R*,R*)]-
- Arformoterol
- (R,R)-Formoterol
- Arformoterol 13D3
- (-)-FORMOTEROL; ARFORMOTEROL; FORMOTEROL
- (R,R)-Formoterol (Arformoterol)
- Formoterol Impurity 10
- Formoterol Impurity 10((R,R)-Formoterol)(Arformoterol)
- ArformoterolQ: What is Arformoterol Q: What is the CAS Number of Arformoterol Q: What is the storage condition of Arformoterol
- N-[2-hydroxy-5-[(1RS) - 1-hydroxy-2-[[(1RS) - 2 - (4-methoxyphenyl) - 1-methylethyl] amino] ethyl] phenyl] formamide
- (R,R)-Formoterol Impurity
- (R,R)-Formoterol Impurity Tartrate
- N-(2-hydroxy-5-((R)-1-hydroxy-2-(((R)-1-(4-methoxyphenyl)propan-2-yl)(methyl)amino)ethyl)phenyl)formamide
- 67346-49-0