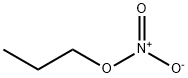
Propylnitrat
|
|
Propylnitrat Eigenschaften
- Schmelzpunkt:
- -99.99°C
- Siedepunkt:
- bp762 110°; bp760 84.8°
- Dichte
- d420 1.0538
- Dampfdruck
- 18 at 20 °C (NIOSH, 1997)
- Brechungsindex
- nD20 1.3979
- L?slichkeit
- Soluble in ether (Weast, 1986) and many alcohols including methanol, ethanol, propanol, and butanol.
- Aggregatzustand
- Clear to yellow liquid
- Henry's Law Constant
- 9.09 at 25 °C (Kames and Schurath, 1992)
- Expositionsgrenzwerte
- NIOSH REL: TWA 25 ppm (109 mg/m3), STEL 40 ppm (170 mg/m3), IDLH 500 ppm; OSHA PEL: TWA 25 ppm; ACGIH TLV: TWA 25 ppm, STEL 40 ppm (adopted).
- EPA chemische Informationen
- n-Propyl nitrate (627-13-4)
Sicherheit
| RIDADR | 1865 | ||
|---|---|---|---|
| OEB | A | ||
| OEL | TWA: 25 ppm (105 mg/m3), STEL: 40 ppm (170 mg/m3) | ||
| HazardClass | 3.1 | ||
| PackingGroup | II | ||
| Giftige Stoffe Daten | 627-13-4(Hazardous Substances Data) | ||
| IDLA | 500 ppm |
Propylnitrat Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
FARBLOSE BIS GELBE FLüSSIGKEIT MIT CHARAKTERISTISCHEM GERUCH.PHYSIKALISCHE GEFAHREN
Die D?mpfe sind schwerer als Luft und k?nnen sich am Boden ausbreiten. Fernzündung m?glich.CHEMISCHE GEFAHREN
Erhitzen kann zu sehr heftiger Verbrennung oder Explosion führen. Bei Sto?, Reibung oder Erschütterung explosionsartige Zersetzung m?glich. Zersetzung beim Verbrennen unter Bildung von Stickstoffoxiden. Starkes Oxidationsmittel. Reagiert sehr heftig mit brennbaren und reduzierenden Stoffen. Reagiert sehr heftig mit starken Oxidationsmitteln.ARBEITSPLATZGRENZWERTE
TLV: 25 ppm (als TWA); 40 ppm (als STEL); BEI vorhanden; (ACGIH 2005).MAK: 25 ppm, 110 mg/m? Spitzenbegrenzung: überschreitungsfaktor II(2); (DFG 2005).
AUFNAHMEWEGE
Aufnahme in den K?rper durch Inhalation der D?mpfe und durch Verschlucken.INHALATIONSGEFAHREN
Beim Verdampfen bei 20°C kann schnell eine gesundheitssch?dliche Kontamination der Luft eintreten.WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION:Die Flüssigkeit reizt die Atemwege, die Augen und die Haut. Inhalation hoher Konzentrationen führt zu Auswirkungen auf das Blut mit nachfolgender Meth?moglobinbildung. Die Auswirkungen treten u.U. verz?gert ein. ?rztliche Beobachtung notwendig (s.Anm.).
LECKAGE
Belüftung. Zündquellen entfernen. Ausgelaufene Flüssigkeit in abdichtbaren Beh?ltern sammeln. Reste mit Sand oder inertem Absorptionsmittel aufnehmen und an einen sicheren Ort bringen. NICHT in die Kanalisation spülen. Pers?nliche Schutzausrüstung: Umgebungsluftunabh?ngiges Atemschutzger?t.Chemische Eigenschaften
n-Propyl nitrate is a colorless to pale yellow liquid. Ethereal odor.Physikalische Eigenschaften
Colorless to light yellow, flammable liquid with an ether-like odor. Odor threshold concentration is 50 ppm (quoted, Amoore and Hautala, 1983).Verwenden
Fuel ignition promoter, in rocket fuel formulations, as organic intermediate.Allgemeine Beschreibung
A white to straw-colored liquid with an ether-like odor. About the same density as water and insoluble in water. Flash point 70°F. Vapors heavier than air. Used as a fuel. Shock sensitive. The shock sensitivity is removed by addition of 1-2% of propane, butane, chloroform, ethyl ether, or methyl ether.Air & Water Reaktionen
Highly flammable. Insoluble in water.Reaktivit?t anzeigen
Organonitrates, such as n-Propyl nitrate, range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. Nitroalkanes are milder oxidizing agents, but still react violently with reducing agents at higher temperature and pressures. Nitroalkanes react with inorganic bases to form explosive salts. The presence of metal oxides increases the thermal sensitivity of nitroalkanes. Nitroalkanes with more than one nitro group are generally explosive. Contact with either strong oxidizers or with combustibles may cause fires and explosions.Hazard
Flammable, severe fire and explosion risk, strong oxidizing material, explosive limits in air 2– 100%. Nausea and headache.Health Hazard
Exposure can cause anoxia and cyanosis. Other effects are weakness, dizziness, and severe headaches.Brandgefahr
Special Hazards of Combustion Products: Toxic gases and vapors, such as oxides of nitrogen and carbon monoxide, may be released in a fire.Sicherheitsprofil
Poison by intravenous route. Inhalation can cause hypotension and methemoglobinemia. Dangerous fire hazard when exposed to heat, flame, or oxidizers. Explosive in the form of vapor when exposed to heat or flame. A shock-sensitive explosive. It can be desensitized by the addition of 1-2% propane, butane, chloroform, dunethyl ether, or dithyl ether. When heated to decomposition it emits toxic fumes of NOx. Used as a fuel ignition promoter, chemical intermediate, and in the manufacture of rocket fuels. See also NITRATES and ESTERS.m?gliche Exposition
Propyl nitrate has been used as an intermediate as a rocket propellant and as an ignition improver in diesel fuels.Versand/Shipping
UN1865 n-Propyl nitrate, Hazard Class: 3; Labels: 3-Flammable liquid.Inkompatibilit?ten
Vapor may form explosive mixture with air. Reacts with reducing agents, combustible materials; may be violent. A shock-sensitive explosive. The shock sensitivity is removed by addition of 1%?2% of propane, butane, chloroform, ethyl ether, or methyl ether . May explode on heating. Forms explosive mixtures with com- bustible materials. This material is an organonitrate. They can range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides, and nitrides, they may begin a vigorous reaction that culminates in a detonation. Nitroalkanes are milder oxidizing agents, but still react violently with reducing agents at higher tem- perature and pressures. Nitroalkanes react with inorganic bases to form explosive salts. The presence of metal oxides increases the thermal sensitivity of nitroalkanes. Nitroalkanes with more than one nitro group are generally explosive. Contact with either strong oxidizers or with combustibles may cause fires and explosions .Waste disposal
Incineration: large quantities of material may require nitrogen oxide removal by catalytic or scrubbing processes . An alternative route suggested involves pouring over soda ash, neutralizing with HCl and flushing to the drain with water.Propylnitrat Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Propylnitrat Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 20)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 |
1026@dideu.com | China | 8670 | 58 |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8615195660023 |
sales@senfeida.com | China | 23046 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 |
sale@mainchem.com | China | 32343 | 55 |
| SelectLab Chemicals GmbH | +49-(0)2383-919350 |
info@selectlab.de | Germany | 394 | 55 |
| Energy Chemical | 021-58432009 400-005-6266 |
marketing@energy-chemical.com | China | 44918 | 58 |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 15229059051 |
1027@dideu.com | China | 10011 | 58 |
| LGC Science (Shanghai) Ltd. | 17717235263 |
cindy.yang@lgcgroup.com | China | 17730 | 58 |
| Shanghai Baoyang Baoxin Biotechnology Co., LTD | 18019222030 18019222030 |
bxsw_sh@163.com | China | 3783 | 58 |
627-13-4(Propylnitrat)Verwandte Suche:
Isopropylnitrat
Cyclohexylnitrat
Isobutylnitrat
Propylnitrat
Glycerintrinitrat
Pentaerithrityltetranitrat
2-Ethylhexylnitrat
Butylnitrat
Isosorbidmononitrat
Isopentylnitrat
Pentylnitrat
3,3,4,4,5,5,6,6,6-Nonafluorhexylnitrat
- nitricacidpropylester
- m-Propyl nitrate
- Nitric acid propyl
- n-Propy nitrate
- n-Propyl nitrate,97%
- npn
- Propylester kyseliny dusicne
- propylesterkyselinydusicne
- n-Propyl nitrate
- Monopropyl nitrate
- n-C3H7ONO2
- Nitrate de propyle normal
- nitratedepropylenormal
- nitricacid,propylester
- JNTOKFNBDFMTIV-UHFFFAOYSA-N
- 627-13-4
- C2H3CH2CH2ONO2