2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile
|
|
|
- CAS-Nr.
- 1187595-85-2
- Englisch Name:
- 2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile
- Synonyma:
- 2-[1-(Ethylsulfonyl)-3-azetidinylidene]acetonitrile;Baricitinib-010;Baricitinib Intermediates;Baricitinib intermediate 2;[1-(ethylsulfonyl)azetidin-3-ylidene]acetonitrile;2-(1-ETHYLSULFONY)AZETIDIN-3-YLIDENE)ACETONITRILE;(1-Ethanesulfonyl-azetidin-3-ylidene)-acetonitrile;2- [1- (Ethylsulfonyl) -3-aziridinyl] acetonitrile;2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile;2-[1-(ethanesulfonyl)azetidin-3-ylidene]acetonitrile
- CBNumber:
- CB62715957
- Summenformel:
- C7H10N2O2S
- Molgewicht:
- 186.23
- MOL-Datei:
- 1187595-85-2.mol
|
2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile Eigenschaften
- Schmelzpunkt:
- 67-69°C
- Siedepunkt:
- 360.8±52.0 °C(Predicted)
- Dichte
- 1.33±0.1 g/cm3(Predicted)
- storage temp.
- Sealed in dry,Room Temperature
- L?slichkeit
- DMSO (Slightly), Methanol (Slightly)
- pka
- -8.49±0.20(Predicted)
- Aggregatzustand
- Solid
- Farbe
- Off-White to Light Yellow
- InChI
- InChI=1S/C7H10N2O2S/c1-2-12(10,11)9-5-7(6-9)3-4-8/h3H,2,5-6H2,1H3
- InChIKey
- HQUIOHSYUKWGOM-UHFFFAOYSA-N
- SMILES
- C(#N)/C=C1\CN(S(CC)(=O)=O)C\1
2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile Chemische Eigenschaften,Einsatz,Produktion Methoden
Verwenden
2-(1-(Ethylsulfonyl)azetidin-3-ylidene)acetonitrile is an intermediate in the synthesis of Baricitinib, a JAK 1 and 2 inhibitor used in the treatment of rheumatoid arthritis.
Synthese
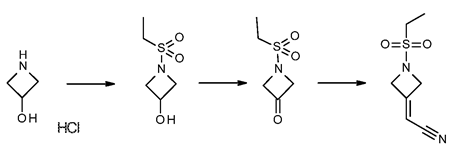
2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile is synthesized by
first treating azetidine-3-ol hydrochloride with an equimolar
equivalent of an alkanesulfonyl chloride, preferably ethanesulfonyl
chloride, to give l-ethylsulfonylazetidin-3-ol. Preferably, the
reaction is performed in a biphasic solution comprising a mixture of an
organic phase and a aqueous phase, preferably THF with an aqueous
solution which is basic, while maintaining the solution at room
temperature or a temperature slightly below room temperature, preferably
20 °C. The reaction is followed to completion using standard monitoring
techniques. Typically, the reaction is complete within 1 to 5 hours.
The organic layer is removed, preferably by distillation, and the
aqueous layer is extracted with an appropriate solvent such as toluene,
p-cymene, and CPME. Preferably the extraction solvent is toluene.
Alternatively, the toluene extractions can be excluded if
recrystallization of 2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile is performed.
2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 284)Lieferanten
- 2-[1-(ethanesulfonyl)azetidin-3-ylidene]acetonitrile
- [1-(ethylsulfonyl)azetidin-3-ylidene]acetonitrile
- 2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile
- 2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile ISO 9001:2015 REACH
- Baricitinib-010
- 2-(1-ETHYLSULFONY)AZETIDIN-3-YLIDENE)ACETONITRILE
- Baricitinib intermediates 2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile
- (1-Ethanesulfonyl-azetidin-3-ylidene)-acetonitrile
- Acetonitrile, 2-[1-(ethylsulfonyl)-3-azetidinylidene]-
- 2-[1-(Ethylsulfonyl)-3-azetidinylidene]acetonitrile
- Baricitinib Intermediates
- 2- [1- (Ethylsulfonyl) -3-azacyclobutyryl] acetonitrile
- 2-[1-(ethyl sulfonyl) -3-azacyclobutyl] acetonitrile
- Baricitinib intermediate 2
- 2- [1- (Ethylsulfonyl) -3-aziridinyl] acetonitrile
- 1187595-85-2
- 1187595-85-2

