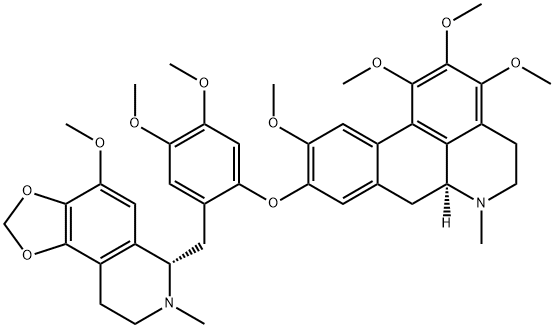
thalmelatidine
|
|
Sicherheit
thalmelatidine Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
The roots of Thalictrum minus yield this highly-substituted bisbenzyliso quinoline alkaloid which is purified by column chromatography followed by recrystallization from MeOH when it yields clusters of colourless needles. The base is dextrorotatory with [α]19D + 47° (CHCl 3 ) and the above structure has been confirmed by synthesis of the alkaloid.Einzelnachweise
Mollov, Thuan., Compt. Rend. Acad. Bulg. Sci., 24,601 (1971)thalmelatidine Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
thalmelatidine Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 1)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| CHEMICAL LAND21 | -- |
sales21@chemicalland21.com | South Korea | 6303 | 74 |
- thalmelatidine
- 9-[4,5-Dimethoxy-2-[(5,6,7,8-tetrahydro-9-methoxy-6-methyl-1,3-dioxolo[4,5-g]isoquinolin-5-yl)methyl]phenoxy]-5,6,6a,7-tetrahydro-1,2,3,10-tetramethoxy-6-methyl-4H-dibenzo[de,g]quinoline
- 4H-Dibenzo[de,g]quinoline, 9-[4,5-dimethoxy-2-[[(6S)-6,7,8,9-tetrahydro-4-methoxy-7-methyl-1,3-dioxolo[4,5-f]isoquinolin-6-yl]methyl]phenoxy]-5,6,6a,7-tetrahydro-1,2,3,10-tetramethoxy-6-methyl-, (6aS)-
- 31199-55-0
- C42H48N2O10