3,4-Dimethoxyphenol Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
S37/39:Bei der Arbeit geeignete Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
Chemische Eigenschaften
Red Crystal Powder
Verwenden
3,4-Dimethoxyphenol is used in the preparation of 5,6-dimethoxy benzofuranone derivatives and multi-target anti Alzheimer compounds. It is also used in the preparation of 3,4-dimethoxyphenyl-beta-D-glucopyranoside and 4-(but-2-enyloxy)-1,2-dimethoxybenzene. Further, it serves as a precursor for 4H-chromenes synthesis.
Synthese
3,4-Dimethoxyphenol is synthesised using Veratraldehyde as a raw material by chemical reaction. The specific synthesis steps are as follows:
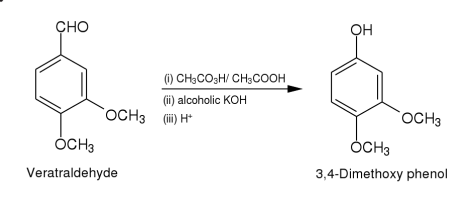
Method (i): To a solution of vertraldehyde (2.11)(5 g,0.03 mole)in glacial acetic
acid (30 ml)is added dropwise to a solution of peracetic acid5(15 ml)
during 30 min.The temperature of the reaction mixture rises,it is kept at
40-45 by cooling.The reaction mixture is left for 10 hr and then
concentrated to about 15 ml in vacuo.The residue is extracted with ether
(2 x 20 ml).The ether layer is distilled.The formate ester of 3,4-
dimethoxyphenol thus obtained is hydrolysed by refluxing with potassium
hydroxide (10 g)in aqueous alcohol (1:4,100 ml)for 1 hr.The reaction
mixture is concentrated in vacuo almost to dryness.It is dissolved in water
(20 ml),the solution rendered acidic with dilute sulphuric acid and
extracted with ether.The ether extract is dried (sodium sulphate)and
distilled.The oily residue thus obtained is subjected to column
chromatography over silica gel(2 x 30 cm).Elution with benzene gave 3,4-dimethoxyphenol as yellow solid.Yield 3g(65.2%).It is crystallised from
benzene as yellow crystalline needles.M.p.78-80(lit.m.p.78-80).
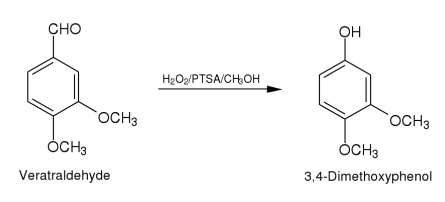
Method (ii): A mixture of veratraldehyde (2.11)(1.66 g,0.01 mole),hydrogen
peroxide (30%,2.6 ml,0.023 mole),p-toluenesulphonic acid (6.88 g,
0.036 mole)and methanol (5 ml)is stirred at room temperature.When the
reaction is complete (as monitored by TLC)the reaction mixture is diluted
with water (50 ml)and extracted with dichloromethane (5 x 50 ml).The
extract is dried (anhydrous sodium sulphate)and purified by column
chromatography over silica gel(2 x 30 cm).Elution with benzene gave 3,4.
dimethoxyphenol as an yellow solid.Yield 1.16 g (75.3%).M.p.78-80.
3,4-Dimethoxyphenol Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Phenol, 3,4-dimethoxy-, 1-formate
3,4,4-TRIMETHOXY-2,5-CYCLOHEXADIEN-1-ONE
2',4',6'-Trihydroxy-3'-methylbutyrophenone
N-(3,4-dimethoxyphenyl)ethanamide
3,4-Dimethoxyphenylboronic acid
4-Allyl-2,6-dimethoxyphenol
4-Hydroxy-3,5-dimethoxybenzaldehyd
3-Methoxycatechin
5-TERT-BUTYLPYROGALLOL
4-Bromveratrol
4'-Hydroxy-3',5'-dimethoxyacetophenon
1-(4-Hydroxy-3-methoxyphenyl)aceton
Downstream Produkte

