2,2'-Bis(trifluoromethyl)benzidine Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R22:Gesundheitssch?dlich beim Verschlucken.
R45:Kann Krebs erzeugen.
R50/53:Sehr giftig für Wasserorganismen, kann in Gew?ssern l?ngerfristig sch?dliche Wirkungen haben.
S-S?tze Betriebsanweisung:
S22:Staub nicht einatmen.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
Beschreibung
2,2'-Bis(trifluoromethyl)benzidine (TFMB) is a fluorinated benzidine derivative with two trifluoromethyl substituents at the 2- and 2'-positions. 2,2'-Bis(trifluoromethyl)benzidine (TFMB) is a rigid and aromatic polyimide used in the synthesis of metal-organic frameworks (MOFs) and covalent organic frameworks (COFs) of high specific surface area.TFMB is also synthesised as UV-resistant and colourless polyimide thin films for optoelectronic applications. TFMB is also used to synthesise UV resistant and colourless polyimide films for optoelectronic applications. The two fluoromethyl groups have a strong electron-withdrawal effect, which reduces the electron dispersion in the π-orbitals of the fully conjugated polymer backbone. This effect results in a much higher transparency of the polyimide film compared to polyimide films containing methyl substituents. This polyimide can be used for the encapsulation of solar cells with MOFs cluster light diffusers.
Verwenden
2,2'-Bis(trifluoromethyl)benzidine is used for producing a high strength flexible transparent polyimide material.
Synthese
This 2,2′-bis(trifluoromethyl)-4,4′-dinitrobiphenyl crystal of 42.5 g, toluene of 127.5 g and 5% Pd/C (AER-TYPE: 50% water contained product) of 1.8 g manufactured by N.E. CHEMCAT Corp. were fed in an autoclave made of stainless steel, after the system was replaced sufficiently with hydrogen, pressured with hydrogen for a reaction pressure to be 1 MPa and reaction was carried out at a reaction temperate of 60 °C for 6 hours. After reaction, catalyst was filtered away, toluene in this reaction liquid was distilled away under reduced pressure, concentrated and crystallized, 2,2′-bis(trifluoromethyl)-4,4′-diaminobiphenyl of 28.5 g (purity converted) was obtained by solid-liquid separation.
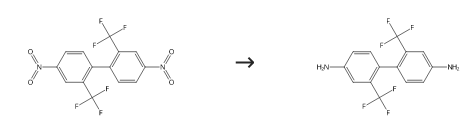
2,2'-Bis(trifluoromethyl)benzidine Upstream-Materialien And Downstream Produkte
Upstream-Materialien
1,1'-Biphenyl, 4,4'-dinitro-2,2'-bis(trifluoromethyl)
Hydrazine, 1,2-bis[3-(trifluoromethyl)phenyl]-
[1,1'-Biphenyl]-2,4'-diamine, 2',4-bis(trifluoromethyl)-
Diazene, bis[3-(trifluoromethyl)phenyl]- (9CI)
2-(Trifluoromethyl)-1-iodo-4-nitrobenzene
4-Nitro-α,α,α-trifluor-o-toluidin
4-NITRO-2-(TRIFLUOROMETHYL)ACETANILIDE
N-[2-(trifluormethyl)phenyl]acetamid
1-Nitro-3-(trifluormethyl)benzol
Downstream Produkte

