3-Methyl-1-nitrobutan Chemische Eigenschaften,Einsatz,Produktion Methoden
synthetische
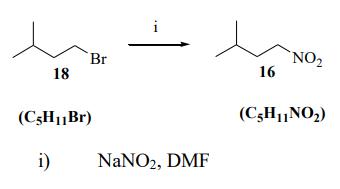
Synthesis of 3-methyl-1-nitrobutane: Isopentyl bromide (120.84 g, 0.80 mol) was added dropwise to a mechanical stirred solution of sodium nitrite (110.39g, 1.60 mol) in DMF (500 mL) at 0°C. The reaction mixture was mechanically stirred overnight at room temperature. Water (1 L) was added to the mixture. The mixture was extracted with pet. ether (4 x 150 mL). The combined organic phases were washed with water (2 x 150 mL). The organic phase was dried with Na2SO4 then the solvent was evaporated under reduced pressure. Distillation under vacuum gave 30.2g (32%) of the colorless nitroalkane 3-methyl-1-nitrobutane (b.p. 58 - 60°C 21 mmHg)
Not visible on TLC IR (neat) ν 2962, 1549, 1470, 1434, 1434, 1381, 1211, 1133, 851 cm-1 . 1H NMR (500 MHz, CDCl3) a 0.96 (d, J = 6.7 Hz, 6 H), 1.69 (h, J = 6.7Hz, 1 H), 1.91 (q, J = 7.2 Hz, 2 H), 4.41 (t, J = 7.3 Hz, 2 H). 13C NMR (125 MHz, CDCl3) a = 21.9, 25.5, 35.9, 74.2
Definition
ChEBI: 3-methyl-1-nitrobutane is a primary nitroalkane that is 2-methylbutane substituted by a nitro group at position 4. It is a herbivore-induced plant volatile found in the leaves of Oenothera biennis. It has a role as a plant metabolite. It is a primary nitroalkane and a volatile organic compound.
3-Methyl-1-nitrobutan Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

