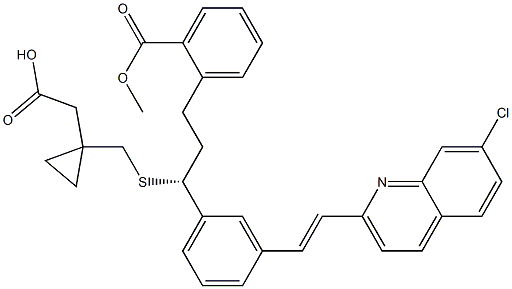
Montelukast Impurity H
|
|
Montelukast Impurity H Eigenschaften
- Schmelzpunkt:
- 55-56°C (dec.)
- Siedepunkt:
- 757.0±60.0 °C(Predicted)
- Dichte
- 1.299±0.06 g/cm3(Predicted)
- storage temp.
- Refrigerator
- L?slichkeit
- Chloroform (Slightly), Methanol (Slightly)
- pka
- 4.76±0.10(Predicted)
- Aggregatzustand
- Solid
- Farbe
- Yellow
Sicherheit
Montelukast Impurity H Chemische Eigenschaften,Einsatz,Produktion Methoden
Chemische Eigenschaften
Yellow SolidMontelukast Impurity H Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Montelukast Impurity H Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 62)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Standardpharm Co. Ltd. | 86-714-3992388 |
overseasales1@yongstandards.com | United States | 14332 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29810 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 |
ada@ipurechemical.com | China | 10319 | 58 |
| CR Corporation Limited | +8613062833949 |
fred.wen@crcorporation.cn | China | 8647 | 58 |
| sgtlifesciences pvt ltd | +8617013299288 |
dj@sgtlifesciences.com | China | 12373 | 58 |
| China National Standard Pharmaceutical Corporation Limited | +8615391658522 |
overseasales@yongstandards.com | China | 11922 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 |
sales@aladdinsci.com | United States | 57505 | 58 |
| Guangzhou CATO Research Chemicals Inc. | +86-020-81960175-617 +8615602392859 |
Intermediate@cato-chem.com | China | 8322 | 58 |
| Moxin Chemicals | +8617320513646 |
mike@molcoo.com | China | 9511 | 58 |
- (R)-2-[1-[[[1-[3-[(E)-2-(7-Ch
- 2-[(3R)-3-[[[1-(CarboxyMethyl)cyclopropyl]Methyl]thio]-3-[3-[(1E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl]propyl]benzoic Acid 1-Methyl Ester
- 2'-Des(1-hydroxy-1-Methylethyl)-2'-Methycarboxy Montelukast
- Montelukast IMpurity H
- Benzoic acid, 2-[(3R)-3-[[[ 1- (carboxyMethyl)cyclopropyl] Methyl]thio]-3-[3-[(1E)-2-(7- chloro-2-quinolinyl)ethenyl] phenyl]propyl]-, 1-Methyl ester
- Montelukast EP Impurity H
- (R,E)-2-(1-(((1-(3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-(2-(methoxycarbonyl)phenyl)propyl)thio)methyl)cyclopropyl)acetic acid
- Montelukast Sodium EP impurity H
- Montelukast Impurity 8(EP Impurity H)
- Montelukast Impurity 8(Montelukast EP Impurity H)
- [R,E]-1-[[[1-[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(methoxycarbonyl)phenyl]propyl]thio]methyl]-cyclopropane acetic acid
- Montelukast Ester Impurity
- Montelukast impurity 11/Montelukast EP Impurity H/Montelukast Methoxycarbonyl Analog/2-[1-[[[(1R)-1-[3-[(1E)-2-(7-Chloroquinolin-2-yl)ethenyl]phenyl]-3-[2-(methoxycarbonyl)phenyl]propyl]sulfanyl]methyl]cyclopropyl]acetic acid
- USP Montelukast Methoxycarbonyl Analog
- Montelukast EP Impurity H (Montelukast Methoxycarbonyl Analog)
- [1-[[[(1R)-1-[3-[(E)-2-(7-Chloroquinolin-2-yl)ethenyl]phenyl]-3-[2-(methoxycarbonyl)phenyl]propyl]sulfanyl] methyl]cyclopropyl]acetic Acid
- 851755-56-1
- Aromatics
- Impurities
- Intermediates & Fine Chemicals
- Pharmaceuticals
- Sulfur & Selenium Compounds