(E)-p-Methoxycinnamsure
|
|
|
- CAS-Nr.
- 943-89-5
- Bezeichnung:
- (E)-p-Methoxycinnamsure
- Englisch Name:
- 4-METHOXYCINNAMIC ACID
- Synonyma:
- PMCA;AKOS B000181;AKOS BB/0034;AKOS BBS-00000769;TIMTEC-BB SBB005717;RARECHEM BK HC T257;OTAVA-BB BB0123400502;4-Methoxycinnamic acid;LABOTEST-BB LT00108121;O-METHYL-P-CUMARIC ACID
- CBNumber:
- CB1152939
- Summenformel:
- C10H10O3
- Molgewicht:
- 178.18
- MOL-Datei:
- 943-89-5.mol
|
(E)-p-Methoxycinnamsure Eigenschaften
(E)-p-Methoxycinnamsure Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S37/39:Bei der Arbeit geeignete Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
Verwenden
Esters derived from trans-4-methoxycinnamic acid are effective absorbers of UV radiation. The starting material was trans-4-methoxycinnamic acid and 3,5dimethoxybenzoic acid methyl ester in isolated avenanthramide alkaloids synthsis. trans-4-Methoxycinnamic acid was converted into its acid chloride (yield 98%) with thionyl chloride. Employed in organic synthesis of pharmaceutical intermediates, synthetic anti-adrenergic drugs esmolol, cosmetics ultraviolet absorption.
synthetische
4-methoxycinnamic acid synthesis : In a 25-mL, roundbottomed flask, 4-methoxybenzaldehyde (0.804 mL, 6.61 mmol) (3), malonic acid (1.75 g, 16.8 mmol), and β-alanine (0.10 g, 1.12 mmol) were dissolved in pyridine (3.0 mL, 37.1 mmol) and heated under reflux for 90 minutes. After cooling to room temperature, the reaction mixture was placed in an ice bath and 8.0 mL of concentrated HCl was slowly added. The resulting white precipitate was collected by vacuum filtration, washed with cold water (2 x 10mL) and dried thoroughly. The product was recrystallized from absolute ethanol (~20 mL).
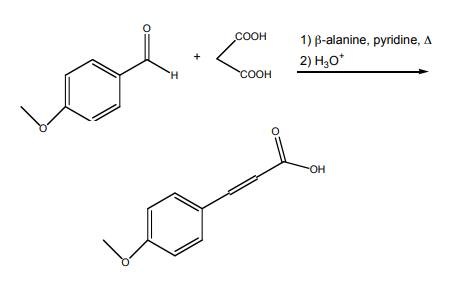
Synthesis of 4-methoxycinnamic acid
l?uterung methode
Crystallise the acid from MeOH to constant melting point and UV spectrum. [Beilstein 10 IV 1005.]
(E)-p-Methoxycinnamsure Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
(E)-p-Methoxycinnamsure Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 215)Lieferanten
943-89-5((E)-p-Methoxycinnamsure)Verwandte Suche:
- 4-METHOXYCINNAMIC ACID ABOUT 98%
- trans-4-Methoxycinnamic acid, 98+%
- (E)-4-Methoxycinnamic acid
- 4-Methoxy-trans-cinnamic acid
- p-Methoxy-trans-cinnamic acid
- 4-Methoxycinnamic acid >=98.0% (GC)
- trans-4-MethoxyClnnamic acid
- 4-Methoxycinnamic acid
- RARECHEM BK HC T257
- TIMTEC-BB SBB005717
- PMCA
- O-METHYL-P-COUMARIC ACID
- O-METHYL-P-CUMARIC ACID
- OTAVA-BB BB0123400502
- TRANS-3-(4-METHOXYPHENYL)ACRYLIC ACID
- METHOXYCINNAMIC ACID,4-
- AKOS B000181
- AKOS BB/0034
- AKOS BBS-00000769
- LABOTEST-BB LT00108121
- (E)-3-(4-METHOXYPHENYL)ACRYLIC ACID
- 3-(4-METHOXY-PHENYL)-ACRYLIC ACID
- (2E)-3-(4-METHOXYPHENYL)ACRYLIC ACID
- (E)-p-methoxycinnamic acid
- 2-Propenoic acid, 3-(4-methoxyphenyl)-, (2E)-
- 2-Propenoic acid, 3-(4-methoxyphenyl)-, (E)-
- (E)-4-MethoxycinnamicAci
- trans-O-Methyl-p-coumaric Acid
- (E)-3-(4-methoxyphenyl)prop-2-enoic acid
- 943-89-5 RARECHEM BK HC T257
- (E)-3-(4-Methoxyphenyl)acrylic acid (compound 3)
- 943-89-5
- 786-13-1
- Exciton Chirality CD Method (for Hydroxyl Groups)
- Absolute Conefiguration Determination (Exciton Chirality CD Method)
- Chalcones, etc. (Building Blocks for Liquid Crystals)
- Enantiomer Excess & Absolute Conefiguration Determination
- Building Blocks for Liquid Crystals
- Carboxylic Acids
- Carbonyl Compounds
- C10
- Building Blocks
- Organic Building Blocks
- Benzenes
- 943-89-5

