Isonicotinonitril Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R20/21/22:Gesundheitssch?dlich beim Einatmen,Verschlucken und Berührung mit der Haut.
S-S?tze Betriebsanweisung:
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
S24/25:Berührung mit den Augen und der Haut vermeiden.
Chemische Eigenschaften
beige solid
Verwenden
4-Cyanopyridine is used as an intermediate in organic synthesis and pharmaceutical substances like isonicotinylhydrazide, which is used in the treatment of tuberculosis. It is used as a precursor for the preparation of isonicotinic acid and 4-diemthylaminopyridine (DMAP). It is involved in the synthesis of 6-methyl-2-pyridin-4-yl-pyrimidin-4-ylamine by reacting with acetonitrile.
Synthese
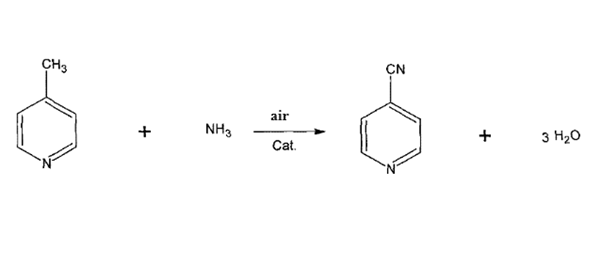
4-Cyanopyridine is synthesised by the reaction of 4-methylpyridine with ammonia and air in the presence of a catalyst. The specific synthesis steps are as follows:
4-Methylpyridine and ammonia vaporisation preheating to 180-330°C, then into the mixing tank and air mixing uniformly, into the fixed-bed reactor by the top of the fixed-bed reactor after the distribution of the catalyst-filled reactor, the control of the reaction temperature in the range of 330-450 ° C, the reaction head pressure is controlled in the range of 0.020-0.070 KPa, the reaction temperature is controlled by molten salts, the reaction gas after the end of the reaction After the reaction, the reaction gas was condensed to sub-zero fractionation to obtain the crude product of 4-cyanopyridine, and the crude product was distilled to obtain the finished product of 4-cyanopyridine. The conversion rate of 4-methylpyridine was above 99%, and the yield of 4-cyanopyridine was above 98%.
m?gliche Exposition
Limits in Air
NIOSH IDLH525 mg/m3
NIOSH REL: (nitriles) 2 ppm, Ceiling Concentration, not
to be exceeded in any 15-minute work period.
Versand/Shipping
UN3276 Nitriles, liquid, toxic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required, Potential Inhalation Hazard (Special
Provision 5).
Inkompatibilit?ten
Oxidizing agents, such as perchlorates,
peroxides, and permanganates. Nitriles may polymerize in
the presence of metals and some metal compounds. They
are incompatible with acids; mixing nitriles with strong
oxidizing acids can lead to extremely violent reactions.
Nitriles are generally incompatible with other oxidizing
agents such as peroxides and epoxides. The combination of
bases and nitriles can produce hydrogen cyanide. Nitriles
are hydrolyzed in both aqueous acid and base to give car-
boxylic acids (or salts of carboxylic acids). These reactions
generate heat. Peroxides convert nitriles to amides. Nitriles
can react vigorously with reducing agents. Acetonitrile and
propionitrile are soluble in water, but nitriles higher than
propionitrile have low aqueous solubility. They are also
insoluble in aqueous acids
.
Isonicotinonitril Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

