6-Methoxy-1,2,3,4-tetrahydronaphthalin-1-on Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R22:Gesundheitssch?dlich beim Verschlucken.
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
R20/21/22:Gesundheitssch?dlich beim Einatmen,Verschlucken und Berührung mit der Haut.
S-S?tze Betriebsanweisung:
S24/25:Berührung mit den Augen und der Haut vermeiden.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
Chemische Eigenschaften
yellow to light brown fine crystalline powder
Verwenden
6-Methoxy-1-tetralone is a reagent useful in the synthesis of (2-(furanyl)vinyl)-1-tetralone chalcones which possesses anticancer agents and inducers of apoptosis.
synthetische
(1) Reacting anisole with an acylating agent in Lewis acid and a solvent at the temperature of-10-40 ℃ to generate an intermediate 1, wherein the molar ratio of the Lewis acid to the acylating agent to the anisole is 1-10: 1-10: 1, the Lewis acid is selected from one or more of concentrated sulfuric acid, phosphoric acid, polyphosphoric acid, zinc chloride, aluminum trichloride, superacid and heteropoly acid;
(2) the intermediate 1 does not need to be separated, the temperature is raised to 70-120 ℃, and the intermediate 1 continues to react to generate 6-methoxy-1-tetralone;
(3) cooling the reaction product, adding water to stop the reaction, extracting, purifying and desolventizing to obtain a crude product of the 6-methoxy-1-tetralone, and refining the crude product by a solvent to obtain a high-purity product.
Synthesis Reference(s)
Journal of the American Chemical Society, 64, p. 94, 1942
DOI: 10.1021/ja01253a025Tetrahedron Letters, 9, p. 2917, 1968
DOI: 10.1016/S0040-4039(00)89611-2
Synthese
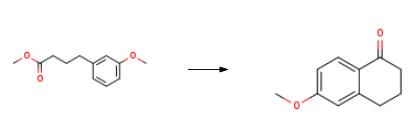
The synthesis of 6-methoxy-1-tetralone is as follows:Eaton's reagent (1.00 mL, 5.31 mmol) was added slowly to a stirred solution of methyl 4-(3-methoxyphenyl)butanoate (4; 0.37g, 1.78 mmol) in DCE (1 mL). The resulting mixture was stirred at 75 °C for 2h under N
2 atmosphere. Then, the reaction mixture was allowed to reach room temperature, and was poured over ice-water and extracted with EtOAc (3 × 15 mL). The combined organic extracts were washed successively with brine (2 × 15 mL) and H
2O (2 × 20 mL), filtered over Na
2SO
4 and concentrated under reduced pressure. The resulting brownish oil was fractionated by column chromatography (silica gel, EtOAc-hexanes, 1:9) to obtain 6-methoxy-1-tetralone as ayellowish oil; yield: 0.28 g (91%).
6-Methoxy-1,2,3,4-tetrahydronaphthalin-1-on Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
17-Ethinyl-3,17-dihydroxy-18-methylestra-2,5(10)-diene3-methylether
3-Methoxy-18-methylestra-1,3,5(10),8-tetraen-17-ethylene ketal
17-Ethinyl-17-hydroxy-18-methylestra-5(10),9(11)-dien-3-one-3-ethylene ketal
3-Methoxy-18-methylestra-2,5(10)dien-17-one 17-ethylene ketal
6-CYANO-1-TETRALONE
6-(benzyloxy)-3,4-dihydronaphthalen-1(2H)-one
4-Methoxyphthalsure
2-Methoxy-6,7,8,9-tetrahydrobenzocyclohepten-5-one
2-[2-(6-methoxytetralin-1-ylidene)-ethyl]-2-methylcyclopentane-1,3-dione
1-CHLORO-6-METHOXY-3,4-DIHYDRO-NAPHTHALENE-2-CARBALDEHYDE
7-METHOXY-4,5-DIHYDRO-NAPHTHO[1,2-D]THIAZOL-2-YLAMINE

